Risk Factors and Attack Rates of Seasonal Influenza Infection: Results of the Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) Seroepidemiologic Cohort Study
- PMID: 30016464
- PMCID: PMC9006182
- DOI: 10.1093/infdis/jiy443
Risk Factors and Attack Rates of Seasonal Influenza Infection: Results of the Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) Seroepidemiologic Cohort Study
Abstract
Background: Understanding the attack rate of influenza infection and the proportion who become ill by risk group is key to implementing prevention measures. While population-based studies of antihemagglutinin antibody responses have been described previously, studies examining both antihemagglutinin and antineuraminidase antibodies are lacking.
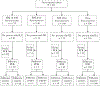
Methods: In 2015, we conducted a seroepidemiologic cohort study of individuals randomly selected from a population in New Zealand. We tested paired sera for hemagglutination inhibition (HAI) or neuraminidase inhibition (NAI) titers for seroconversion. We followed participants weekly and performed influenza polymerase chain reaction (PCR) for those reporting influenza-like illness (ILI).
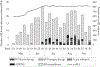
Results: Influenza infection (either HAI or NAI seroconversion) was found in 321 (35% [95% confidence interval, 32%-38%]) of 911 unvaccinated participants, of whom 100 (31%) seroconverted to NAI alone. Young children and Pacific peoples experienced the highest influenza infection attack rates, but overall only a quarter of all infected reported influenza PCR-confirmed ILI, and one-quarter of these sought medical attention. Seroconversion to NAI alone was higher among children aged <5 years vs those aged ≥5 years (14% vs 4%; P < .001) and among those with influenza B vs A(H3N2) virus infections (7% vs 0.3%; P < .001).
Conclusions: Measurement of antineuraminidase antibodies in addition to antihemagglutinin antibodies may be important in capturing the true influenza infection rates.
Conflict of interest statement
Figures
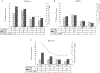
Comment in
-
The Value of Neuraminidase Inhibition Antibody Titers in Influenza Seroepidemiology.J Infect Dis. 2019 Jan 9;219(3):341-343. doi: 10.1093/infdis/jiy446. J Infect Dis. 2019. PMID: 30011035 No abstract available.
References
-
- Cox NJ, Subbarao K. Influenza. Lancet 1999; 354:1277–82. - PubMed
-
- Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther 2011; 9:669–83. - PubMed
-
- Dingle JH, Badger GF, Feller AE, Hodges RG, Jordan WS Jr, Rammelkamp CH Jr. A study of illness in a group of Cleveland families. I. Plan of study and certain general observations. Am J Hyg 1953; 58:16–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

