Antibody Fucosylation Lowers the FcγRIIIa/CD16a Affinity by Limiting the Conformations Sampled by the N162-Glycan
- PMID: 30016589
- PMCID: PMC6415948
- DOI: 10.1021/acschembio.8b00342
Antibody Fucosylation Lowers the FcγRIIIa/CD16a Affinity by Limiting the Conformations Sampled by the N162-Glycan
Abstract
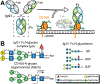
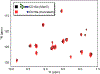
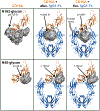
Therapeutic monoclonal antibodies (mAbs) are largely based on the immunoglobulin G1 (IgG1) scaffold, and many elicit a cytotoxic cell-mediated response by binding Fc γ receptors. Core fucosylation, a prevalent modification to the asparagine (N)-linked carbohydrate on the IgG1 crystallizable fragment (Fc), decreases the Fc γ receptor IIIa (CD16a) binding affinity and mAb efficacy. We determined IgG1 Fc fucosylation reduced the CD16a affinity by 1.7 ± 0.1 kcal/mol when compared to that of afucosylated IgG1 Fc; however, CD16a N-glycan truncation decreased this penalty by 1.2 ± 0.1 kcal/mol or 70%. Fc fucosylation restricted the manifold of conformations sampled by displacing the CD16a Asn162-glycan that impinges upon the linkage between the α-mannose(1-6)β-mannose residues and promoted contacts with the IgG Tyr296 residue. Fucosylation also impacted the IgG1 Fc structure as indicated by changes in resonance frequencies and nuclear spin relaxation observed by solution nuclear magnetic resonance spectroscopy. The effects of fucosylation on IgG1 Fc may account for the remaining 0.5 ± 0.1 kcal/mol penalty of fucosylated IgG1 Fc binding CD16a when compared to that of afucosylated IgG1 Fc. Our results indicated the CD16a Asn162-glycan modulates the antibody affinity indirectly by reducing the volume sampled, as opposed to a direct mechanism with intermolecular glycan-glycan contacts previously proposed to stabilize this system. Thus, antibody engineering to enhance intermolecular glycan-glycan contacts will likely provide limited improvement, and future designs should maximize the affinity by maintaining the CD16a Asn162-glycan conformational heterogeneity.
Figures

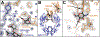
References
-
- Bakema JE, and van Egmond M (2014) Fc receptor-dependent mechanisms of monoclonal antibody therapy of cancer, Curr. Top. Microbiol. and Immunol. 382, 373–392. - PubMed
-
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, and Watier H (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor Fc gamma RIIIa gene, Blood 99, 754–758. - PubMed
-
- Weng WK, and Levy R (2003) Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma, J. Clin. Oncol. 21, 3940–3947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

