Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET-Rearranged Lung Cancers
- PMID: 30017832
- PMCID: PMC6434708
- DOI: 10.1016/j.jtho.2018.07.004
Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET-Rearranged Lung Cancers
Abstract
Introduction: In ret proto-oncogene (RET)-rearranged lung cancers, data on the frequency of brain metastases and, in particular, the outcomes of multikinase inhibitor therapy in patients with intracranial disease are not well characterized.
Methods: A global, multi-institutional registry (cohort A, n = 114) and a bi-institutional data set (cohort B, n = 71) of RET-rearranged lung cancer patients were analyzed. Patients were eligible if they had stage IV lung cancers harboring a RET rearrangement by local testing. The incidence of brain metastases and outcomes with multikinase inhibitor therapy were determined.
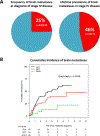
Results: The frequency of brain metastases at the time of diagnosis of stage IV disease was 25% (95% confidence interval [CI]: 18%-32%) in all patients from both cohorts. The lifetime prevalence of brain metastasis in stage IV disease was 46% (95% CI: 34%-58%) in patients for whom longitudinal data was available. The cumulative incidence of brain metastases was significantly different (p = 0.0039) between RET-, ROS1-, and ALK receptor tyrosine kinase (ALK)-rearranged lung cancers, with RET intermediate between the other two groups. Although intracranial response data was not available in cohort A, the median progression-free survival of multikinase inhibitor therapy (cabozantinib, vandetanib, or sunitinib) in patients with brain metastases was 2.1 months (95% CI: 1.3-2.9 months, n = 10). In cohort B, an intracranial response was observed in 2 of 11 patients (18%) treated with cabozantinib, vandetanib (± everolimus), ponatinib, or alectinib; the median overall progression-free survival (intracranial and extracranial) was 3.9 months (95% CI: 2.0-4.9 months).
Conclusions: Brain metastases occur frequently in RET-rearranged lung cancers, and outcomes with multikinase inhibitor therapy in general are suboptimal. Novel RET-directed targeted therapy strategies are needed.
Keywords: RET fusion; RET rearrangement; brain metastases; lung cancer cabozantinib; vandetanib multikinase inhibitor.
Copyright © 2018 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURES
No Conflicts of interest were reported for: Jessica J. Lin, Thomas Filleron, Ai Ni, Julie Milia, Isabella Bergagnini, Vaios Hatzoglou, Vamsidhar Velcheti, Michael Offin, Julien Mazieres, David P. Carbone, Benjamin Besse, Tony Mok, Mark M. Awad, Dwight Owen, Ross Camidge, Nir Peled and Oliver Gautschi.
Figures
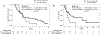
References
-
- Yoh K, Seto T, Satouchi M, et al.: Vandetanib in patients with previously treated RET- rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med, 2016 - PubMed
-
- Lee SH, Lee JK, Ahn MJ, et al.: Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 28:292–297, 2017 - PubMed
-
- Velcheti VTH KL Reckamp JC. Yang H. Nokihara P Sachdev K. Feit T. Kubota T. Nakada CE. Dutcus M Ren T. Tamura Phase 2 study of lenvatinib (LN) in patients (Pts) with RET fusion-positive adenocarcinoma of the lung. ESMO Congress:1204PD, 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

