Extracellular Vesicles Containing IL-4 Modulate Neuroinflammation in a Mouse Model of Multiple Sclerosis
- PMID: 30017878
- PMCID: PMC6127510
- DOI: 10.1016/j.ymthe.2018.06.024
Extracellular Vesicles Containing IL-4 Modulate Neuroinflammation in a Mouse Model of Multiple Sclerosis
Abstract
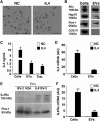
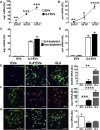
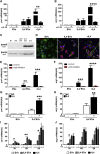
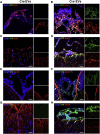
Extracellular vesicles (EVs) play a major role in cell-to-cell communication in physiological and pathological conditions, and their manipulation may represent a promising therapeutic strategy. Microglia, the parenchymal mononuclear phagocytes of the brain, modulate neighboring cells also through the release of EVs. The production of custom EVs filled with desired molecules, possibly targeted to make their uptake cell specific, and their administration in biological fluids may represent a valid approach for drug delivery. We engineered a murine microglia cell line, BV-2, to release EVs overexpressing the endogenous "eat me" signal Lactadherin (Mfg-e8) on the surface to target phagocytes and containing the anti-inflammatory cytokine IL-4. A single injection of 107 IL-4+Mfg-e8+ EVs into the cisterna magna modulated established neuroinflammation and significantly reduced clinical signs in the mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE). Injected IL-4+Mfg-e8+ EVs target mainly phagocytes (i.e., macrophages and microglia) surrounding liquoral spaces, and their cargo promote the upregulation of anti-inflammatory markers chitinase 3-like 3 (ym1) and arginase-1 (arg1), significantly reducing tissue damage. Engineered EVs may represent a biological drug delivery tool able to deliver multiple functional molecules simultaneously to treat neuroinflammatory diseases.
Keywords: extracellular vesicles; neuroinflammation; phagocytes.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. - PubMed
-
- Porro C., Trotta T., Panaro M.A. Microvesicles in the brain: Biomarker, messenger or mediator? J. Neuroimmunol. 2015;288:70–78. - PubMed
-
- Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

