Propofol inhibits prokaryotic voltage-gated Na+ channels by promoting activation-coupled inactivation
- PMID: 30018038
- PMCID: PMC6122921
- DOI: 10.1085/jgp.201711924
Propofol inhibits prokaryotic voltage-gated Na+ channels by promoting activation-coupled inactivation
Abstract
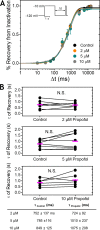
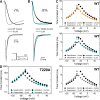
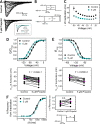
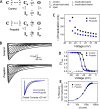
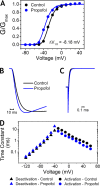
Propofol is widely used in the clinic for the induction and maintenance of general anesthesia. As with most general anesthetics, however, our understanding of its mechanism of action remains incomplete. Local and general anesthetics largely inhibit voltage-gated Na+ channels (Navs) by inducing an apparent stabilization of the inactivated state, associated in some instances with pore block. To determine the biophysical and molecular basis of propofol action in Navs, we investigated NaChBac and NavMs, two prokaryotic Navs with distinct voltage dependencies and gating kinetics, by whole-cell patch clamp electrophysiology in the absence and presence of propofol at clinically relevant concentrations (2-10 µM). In both Navs, propofol induced a hyperpolarizing shift of the pre-pulse inactivation curve without any significant effects on recovery from inactivation at strongly hyperpolarized voltages, demonstrating that propofol does not stabilize the inactivated state. Moreover, there was no evidence of fast or slow pore block by propofol in a non-inactivating NaChBac mutant (T220A). Propofol also induced hyperpolarizing shifts of the conductance-voltage relationships with negligible effects on the time constants of deactivation at hyperpolarized voltages, indicating that propofol does not stabilize the open state. Instead, propofol decreases the time constants of macroscopic activation and inactivation. Adopting a kinetic scheme of Nav gating that assumes preferential closed-state recovery from inactivation, a 1.7-fold acceleration of the rate constant of activation and a 1.4-fold acceleration of the rate constant of inactivation were sufficient to reproduce experimental observations with computer simulations. In addition, molecular dynamics simulations and molecular docking suggest that propofol binding involves interactions with gating machinery in the S4-S5 linker and external pore regions. Our findings show that propofol is primarily a positive gating modulator of prokaryotic Navs, which ultimately inhibits the channels by promoting activation-coupled inactivation.
© 2018 Yang et al.
Figures




Comment in
-
Propofol's paradox, explained.J Gen Physiol. 2018 Sep 3;150(9):1231-1232. doi: 10.1085/jgp.201812197. Epub 2018 Aug 16. J Gen Physiol. 2018. PMID: 30115662 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

