Propofol inhibits the voltage-gated sodium channel NaChBac at multiple sites
- PMID: 30018039
- PMCID: PMC6122922
- DOI: 10.1085/jgp.201811993
Propofol inhibits the voltage-gated sodium channel NaChBac at multiple sites
Abstract
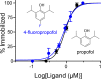
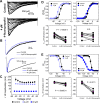
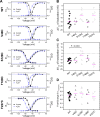
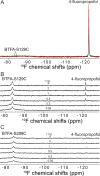
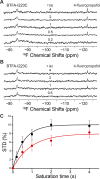
Voltage-gated sodium (NaV) channels are important targets of general anesthetics, including the intravenous anesthetic propofol. Electrophysiology studies on the prokaryotic NaV channel NaChBac have demonstrated that propofol promotes channel activation and accelerates activation-coupled inactivation, but the molecular mechanisms of these effects are unclear. Here, guided by computational docking and molecular dynamics simulations, we predict several propofol-binding sites in NaChBac. We then strategically place small fluorinated probes at these putative binding sites and experimentally quantify the interaction strengths with a fluorinated propofol analogue, 4-fluoropropofol. In vitro and in vivo measurements show that 4-fluoropropofol and propofol have similar effects on NaChBac function and nearly identical anesthetizing effects on tadpole mobility. Using quantitative analysis by 19F-NMR saturation transfer difference spectroscopy, we reveal strong intermolecular cross-relaxation rate constants between 4-fluoropropofol and four different regions of NaChBac, including the activation gate and selectivity filter in the pore, the voltage sensing domain, and the S4-S5 linker. Unlike volatile anesthetics, 4-fluoropropofol does not bind to the extracellular interface of the pore domain. Collectively, our results show that propofol inhibits NaChBac at multiple sites, likely with distinct modes of action. This study provides a molecular basis for understanding the net inhibitory action of propofol on NaV channels.
© 2018 Wang et al.
Figures


Comment in
-
Propofol's paradox, explained.J Gen Physiol. 2018 Sep 3;150(9):1231-1232. doi: 10.1085/jgp.201812197. Epub 2018 Aug 16. J Gen Physiol. 2018. PMID: 30115662 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

