Germline-activating mutations in PIK3CD compromise B cell development and function
- PMID: 30018075
- PMCID: PMC6080914
- DOI: 10.1084/jem.20180010
Germline-activating mutations in PIK3CD compromise B cell development and function
Abstract
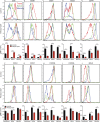
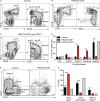
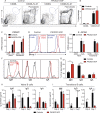
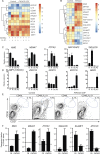
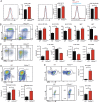
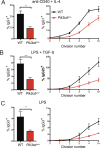
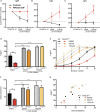
Gain-of-function (GOF) mutations in PIK3CD, encoding the p110δ subunit of phosphatidylinositide 3-kinase (PI3K), cause a primary immunodeficiency. Affected individuals display impaired humoral immune responses following infection or immunization. To establish mechanisms underlying these immune defects, we studied a large cohort of patients with PIK3CD GOF mutations and established a novel mouse model using CRISPR/Cas9-mediated gene editing to introduce a common pathogenic mutation in Pik3cd In both species, hyperactive PI3K severely affected B cell development and differentiation in the bone marrow and the periphery. Furthermore, PI3K GOF B cells exhibited intrinsic defects in class-switch recombination (CSR) due to impaired induction of activation-induced cytidine deaminase (AID) and failure to acquire a plasmablast gene signature and phenotype. Importantly, defects in CSR, AID expression, and Ig secretion were restored by leniolisib, a specific p110δ inhibitor. Our findings reveal key roles for balanced PI3K signaling in B cell development and long-lived humoral immunity and memory and establish the validity of treating affected individuals with p110δ inhibitors.
© 2018 Avery et al.
Figures


References
-
- Aagaard-Tillery K.M., and Jelinek D.F.. 1996. Phosphatidylinositol 3-kinase activation in normal human B lymphocytes. J. Immunol. 156:4543–4554. - PubMed
-
- Agematsu K., Nagumo H., Yang F.C., Nakazawa T., Fukushima K., Ito S., Sugita K., Mori T., Kobata T., Morimoto C., and Komiyama A.. 1997. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur. J. Immunol. 27:2073–2079. 10.1002/eji.1830270835 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

