Analytical and Sample Preparation Protocol for Therapeutic Drug Monitoring of 12 Thiopurine Metabolites Related to Clinical Treatment of Inflammatory Bowel Disease
- PMID: 30018218
- PMCID: PMC6100499
- DOI: 10.3390/molecules23071744
Analytical and Sample Preparation Protocol for Therapeutic Drug Monitoring of 12 Thiopurine Metabolites Related to Clinical Treatment of Inflammatory Bowel Disease
Abstract
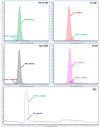
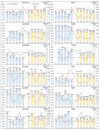
Thiopurines (TP) represent an important therapeutic tool for the treatment of inflammatory bowel diseases (IBD) in the current situation of rising incidence and health care costs. The results of multiple clinical studies aimed at finding correlations between levels of TP metabolites and response of IBD patients to the treatment are, however, often controversial due to variability in analytical and sample preparation procedures among these studies. In this work, therefore, an updated analytical and sample preparation procedure for therapeutic drug monitoring (TDM) of TP metabolites in blood samples obtained from patients with IBD was proposed to establish a unified protocol. An advanced analytical method based on ion-exchange liquid chromatography hyphenated with tandem mass spectrometry (IEC-ESI-MS/MS) was used for the determination of the profiles of 12 individual TP metabolites in the particular steps of sample preparation procedure including blood collection, red blood cells (RBC) isolation, lysis, and storage. Favorable performance parameters of the IEC-ESI-MS/MS method (LLOQs 1⁻10 nmol/L, accuracy 95⁻105%, intra-day and inter-day precision < 10%, selectivity demonstrated via no sample matrix interferences) and acceptable stability (peak area fluctuations < 15%) of clinical samples under the proposed sample preparation conditions {(i) EDTA anticoagulant tube for the blood collection; (ii) 4 °C and 4 h between the sample collection and RBC isolation; (iii) phosphate-buffered saline for RBC washing and re-suspendation; (iv) -20 °C for RBC lysis and short-term storage; (v) 50 mmol/L phosphate buffer, pH 7.4, 10 mmol/L DTT as a stabilizing medium for TPN in RBC lysates} demonstrated the suitability of such protocol for a well-defined and reliable routine use in studies on thiopurines TDM.
Keywords: clinical analysis; comprehensive stability study; high performance liquid chromatography; inflammatory bowel diseases; mass spectrometry; profiling of thiopurines; sample treatment; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Targan S.R., Shanahan F., Karp L.C. Inflammatory Bowel Disease: Translating Basic Science into Clinical Practice. Wiley-Blackwell; Oxford, UK: 2010.
-
- Molodecky N.A., Soon I.N.G.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

