Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence
- PMID: 30018566
- PMCID: PMC6037948
- DOI: 10.3389/fphys.2018.00809
Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence
Abstract
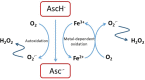
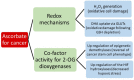
Whether vitamin C (ascorbate) has a role to play as an anti-cancer agent has been debated for decades. Ascorbate has been used by cancer patients in an unregulated environment, either as a dietary supplement or in pharmacological doses administered by infusion, with numerous reports of clinical benefit, but in the absence of rigorous clinical trial data. The design of appropriate clinical trials has been hindered by a lack of understanding of the mechanism(s) of action that would inform the choice of effective dose, timing of administration and likely responsive cancer models. More recently, expanded understanding of the biological activities of ascorbate has led to a number of plausible hypotheses for mechanisms of anti-cancer activity. Prominent among these are the generation of significant quantities of hydrogen peroxide by the autoxidation of supra-physiological concentrations of ascorbate and stimulation of the 2-oxoglutarate-dependent dioxygenase family of enzymes (2-OGDDs) that have a cofactor requirement for ascorbate. Hydrogen peroxide generation is postulated to generate oxidative stress that preferentially targets cancer cells. The 2-OGDDs include the hydroxylases that regulate the hypoxic response, a major driver of tumor survival, angiogenesis, stem cell phenotype and metastasis, and the epigenetic histone and DNA demethylases. The latter are of particular interest, with recent studies suggesting a promising role for ascorbate in the regulation of the ten-eleven translocase (TET) DNA demethylases in hematological cancers. Support for these proposed mechanisms has come from many in vitro studies, and xenograft animal models have consistently shown an anti-cancer effect of ascorbate administration. However, decisive evidence for any particular mechanism(s) of action is not yet available from an in vivo setting. With a number of early phase clinical trials currently underway, evidence for potential mechanism(s) of action is required to inform the most appropriate study design and choice of cancer model. Hopefully such information will result in sound clinical data that will avert adding any further controversy to this already contentious debate.
Keywords: HIF hydroxylases; antioxidant; ascorbate; epigenetic demethylases; hydrogen peroxide; hypoxia-inducible factor; iron-mediated autoxidation; ten-eleven translocases.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

