Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes
- PMID: 30018677
- PMCID: PMC6029458
- DOI: 10.1155/2018/2732017
Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes
Abstract
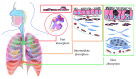
The inhalation route is frequently used to administer drugs for the management of respiratory diseases such as asthma or chronic obstructive pulmonary disease. Compared with other routes of administration, inhalation offers a number of advantages in the treatment of these diseases. For example, via inhalation, a drug is directly delivered to the target organ, conferring high pulmonary drug concentrations and low systemic drug concentrations. Therefore, drug inhalation is typically associated with high pulmonary efficacy and minimal systemic side effects. The lung, as a target, represents an organ with a complex structure and multiple pulmonary-specific pharmacokinetic processes, including (1) drug particle/droplet deposition; (2) pulmonary drug dissolution; (3) mucociliary and macrophage clearance; (4) absorption to lung tissue; (5) pulmonary tissue retention and tissue metabolism; and (6) absorptive drug clearance to the systemic perfusion. In this review, we describe these pharmacokinetic processes and explain how they may be influenced by drug-, formulation- and device-, and patient-related factors. Furthermore, we highlight the complex interplay between these processes and describe, using the examples of inhaled albuterol, fluticasone propionate, budesonide, and olodaterol, how various sequential or parallel pulmonary processes should be considered in order to comprehend the pulmonary fate of inhaled drugs.
Figures


References
-
- Lavorini F., Mannini C., Chellini E. Challenges of inhaler use in the treatment of asthma and chronic obstructive pulmonary disease. EMJ Respiratory. 2015;3:98–105.
-
- Global Initiative for Asthma. GINA Report: Global Strategy for Asthma Management and Prevention. 2017. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-managem...
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2017. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-preve... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

