HIV-associated cardiovascular disease: importance of platelet activation and cardiac fibrosis in the setting of specific antiretroviral therapies
- PMID: 30018781
- PMCID: PMC6045710
- DOI: 10.1136/openhrt-2018-000823
HIV-associated cardiovascular disease: importance of platelet activation and cardiac fibrosis in the setting of specific antiretroviral therapies
Abstract
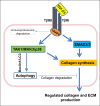
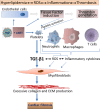
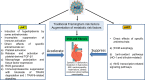
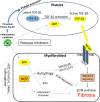
HIV infection is a risk factor for cardiovascular disease (CVD). This risk is accentuated by certain combination antiretroviral therapies (cARTs), independent of their effects on lipid metabolism and insulin sensitivity. We sought to define potential mechanisms for this association through systematic review of clinical and preclinical studies of CVD in the setting of HIV/cART from the English language literature from 1989 to March 2018. We used PubMed, Web of Knowledge and Google Scholar, and conference abstracts for the years 2015-March 2018. We uncovered three themes: (1) a critical role for the HIV protease inhibitor (PI) ritonavir and certain other PI-based regimens. (2) The importance of platelet activation. Virtually all PIs, and one nucleoside reverse transcriptase inhibitor, abacavir, activate platelets, but a role for this phenomenon in clinical CVD risk may require additional postactivation processes, including: release of platelet transforming growth factor-β1; induction of oxidative stress with production of reactive oxygen species from vascular cells; suppression of extracellular matrix autophagy; and/or sustained proinflammatory signalling, leading to cardiac fibrosis and dysfunction. Cardiac fibrosis may underlie an apparent shift in the character of HIV-linked CVD over the past decade from primarily left ventricular systolic to diastolic dysfunction, possibly driven by cART. (3) Recognition of the need for novel interventions. Switching from cART regimens based on PIs to contemporary antiretroviral agents such as the integrase strand transfer inhibitors, which have not been linked to clinical CVD, may not mitigate CVD risk assumed under prior cART. In conclusion, attention to the effects of specific antiretroviral drugs on platelet activation and related profibrotic signalling pathways should help: guide selection of appropriate anti-HIV therapy; assist in evaluation of CVD risk related to novel antiretrovirals; and direct appropriate interventions.
Keywords: HIV; cART; myocardial fibrosis; oxidative stress; platelet activation.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Boyd MA, Mocroft A, Ryom L, et al. . Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study. PLoS Med 2017;14:e1002424 10.1371/journal.pmed.1002424 - DOI - PMC - PubMed
-
- Gallant J, Hsue P, Budd D, et al. . Healthcare utilization and direct costs of noninfectious comorbidities in HIV-infected patients in the USA. Curr Med Res Opin 2017:1383889. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
