Therapeutic benefit of apremilast on enthesitis and dactylitis in patients with psoriatic arthritis: a pooled analysis of the PALACE 1-3 studies
- PMID: 30018799
- PMCID: PMC6045740
- DOI: 10.1136/rmdopen-2018-000669
Therapeutic benefit of apremilast on enthesitis and dactylitis in patients with psoriatic arthritis: a pooled analysis of the PALACE 1-3 studies
Abstract
Objective: The Psoriatic Arthritis Long-term Assessment of Clinical Efficacy (PALACE) clinical trial programme findings demonstrated that apremilast, an oral phosphodiesterase 4 inhibitor, is effective for treating psoriatic arthritis (PsA). Enthesitis and dactylitis are difficult-to-treat features of PsA leading to disability and affecting quality of life. PALACE 1, 2 and 3 data were pooled to assess the efficacy of apremilast on enthesitis and dactylitis outcomes in patients with these conditions at baseline.
Methods: Patients with enthesitis (n=945) or dactylitis (n=633) at baseline were analysed after receiving double-blind treatment with placebo, apremilast 30 mg two times per day or apremilast 20 mg two times per day up to 52 weeks and continuing up to 5 years. Data were analysed through 156 weeks. Enthesitis was evaluated by Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) and dactylitis via dactylitis count.
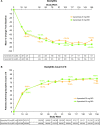
Results: At week 24, patients receiving apremilast 30 mg two times per day demonstrated a significantly greater mean change in enthesitis (-1.3 vs -0.9; p<0.05) and dactylitis (-1.8 vs -1.3; p<0.01) vs placebo. Patients in the 30 mg dose group showed significantly greater mean (-23.6% vs -7.0%; p<0.05) and median (-50.0% vs -21.1%; p<0.05) per cent changes in MASES; mean and median per cent changes in dactylitis count were numerically, but not significantly, different for either apremilast dose in patients with dactylitis. In the patient population remaining on apremilast, observed mean and median improvements in both conditions were sustained through 156 weeks.
Conclusion: Apremilast is effective for the treatment of active PsA, including improvements in enthesitis and dactylitis up to 3 years.
Trial registration numbers: NCT01172938, NCT01212757 and NCT01212770.
Keywords: anti-rheumatic agents; apremilast; arthritis; phosphodiesterase 4 inhibitors; psoriatic.
Conflict of interest statement
Competing interests: DDG has received grant/research support and has served as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene Corporation, Janssen, Novartis, Pfizer and UCB. AK has received grant/research support from Abbott, Amgen, AstraZeneca, BMS, Celgene Corporation, Centocor-Janssen, Pfizer, Roche and UCB. JJG-R has received grant/research support from Roche and Schering-Plough. JW has received research support from and served as a consultant for Abbott, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer and UCB. MC has received research support from and served as a consultant for Actelion, Bristol-Myers Squibb and Sanofi-Aventis. GS has received research support from and served as a consultant for Abbott, Celgene Corporation, Roche and UCB. EL has received research support from and served on a speakers bureau for Amgen, Eli Lilly, Novartis and Servier. ND, BG and LT are employees of Celgene Corporation. CJE has received research support from Celgene Corporation, Pfizer, Roche and Samsung and has served on a speakers bureau for Abbott, GlaxoSmithKline, Pfizer and Roche. CAB has received research support from Amgen, Bristol-Myers Squibb, Incyte, Eli Lilly, Merck and Pfizer. PJM has received research support from and served as a consultant for Abbott, Amgen, Biogen Idec, Bristol-Myers Squibb, Celgene Corporation, Eli Lilly, Genentech, Janssen, Novartis, Pfizer, Roche and UCB and has served on a speakers bureau for Abbott, Amgen, Biogen Idec, Bristol-Myers Squibb, Eli Lilly, Genentech, Janssen, Pfizer and UCB.
Figures

References
-
- Carneiro S, Bortoluzzo A, Goncalves C. Effect of enthesitis on 1505 Brazilian patients with spondyloarthritis. J Rheumatol 2013;40:1725. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
