Synovial Predictors of Differentiation to Definite Arthritis in Patients With Seronegative Undifferentiated Peripheral Inflammatory Arthritis: microRNA Signature, Histological, and Ultrasound Features
- PMID: 30018954
- PMCID: PMC6037719
- DOI: 10.3389/fmed.2018.00186
Synovial Predictors of Differentiation to Definite Arthritis in Patients With Seronegative Undifferentiated Peripheral Inflammatory Arthritis: microRNA Signature, Histological, and Ultrasound Features
Abstract
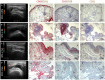
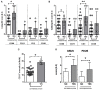
Objectives: To examine synovial tissue (ST) predictors of clinical differentiation in patients with seronegative undifferentiated peripheral inflammatory arthritis (UPIA). Methods: Fourty-two patients with IgA/IgM-Rheumatoid Factor and anti-citrullinated peptide antibodies negative UPIA, naive to Disease-Modifying Anti-Rheumatic Drugs, underwent Gray Scale (GSUS) and power Doppler (PDUS) evaluation and Ultrasound (US) guided ST biopsy. CD68, CD3, CD21, CD20, and CD31 synovial expression was evaluated by immunohistochemistry. Whole ST microRNA expression was assessed using miScript miRNA PCR Array. Peripheral blood (PB) and synovial fluid (SF) IL-6, VEGF-A, and VEGF-D levels were measured by ELISA and ST TNF expression was assessed by RT-PCR. Each patient was prospectively monitored and classified at baseline and within 1 year as UPIA, Rheumatoid Arthritis (RA), Spondyloarthritis (SpA) or Psoriatic Arthritis (PsA), respectively. Results: At baseline, CD68+ cells were the most common cells within the lining layer (p < 0.001) in seronegative UPIA, directly correlating with GSUS (R = 0.36; p = 0.02) and PDUS (R = 0.55; p < 0.001). Synovial CD31+ vessels count directly correlated with GSUS (R = 0.41; p = 0.01) and PDUS (R = 0.52; p < 0.001). During the follow-up, 6 (14.3%) UPIA reached a definite diagnosis (2 RA, 2 SpA and 2 PsA, respectively). At baseline, UPIA who differentiated had higher GSUS (p = 0.01), PDUS scores (p = 0.02) and higher histological scores for CD68+ (p = 0.005 and p = 0.04 for lining and sublining respectively), sublining CD3+ cells (p = 0.002), CD31+ vessels count (p < 0.001) and higher IL-6 PB levels (p = 0.01) than patients who remained as UPIA. MiRNA PCR Array showed that among the 86 tested miRNA species, at baseline, miR-346 and miR-214 were significantly down-regulated (p = 0.02 for both) in ST of UPIA who differentiated than in patients who remained as UPIA, inversely correlating with the lining CD68+ cells IHC score (R = -0.641; p = 0.048) and CD31+ vessels count (R = -0.665; p = 0.036) and with higher baseline ST expression of TNF (p = 0.014). Finally, logistic regression analysis demonstrated that baseline GSUS and PDUS scores ≥1.5 [OR:22.93 (95%CI:0.98-534.30)] and CD31+ vessels count ≥24.3 [OR:23.66 (95%CI:1.50-373.02)] were independent factors associated with the development of definite arthritis. Conclusions: MiRNA signature, histological and US features of ST may help in the identification of seronegative UPIA with high likelihood of clinical differentiation toward definite seronegative arthritis.
Keywords: microRNA; predictor; synovial tissue biopsy; ultrasonography; undifferentiated peripheral inflammatory arthritis.
Figures



References
-
- Machado P, Castrejon I, Katchamart W, Koevoets R, Kuriya B, Schoels M, et al. . Multinational evidence-based recommendations on how to investigate and follow-up undifferentiated peripheral inflammatory arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3e Initiative. Ann Rheum Dis. (2011) 70:15–24. 10.1136/ard.2010.130625 - DOI - PMC - PubMed
-
- Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. . The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheumatol. (2000) 43:155–63. 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3 - DOI - PubMed
-
- Nielen MM, van der Horst AR, van Schaardenburg D, van der Horst-Bruinsma IE, van de Stadt RJ, Aarden L, et al. . Antibodies to citrullinated human fibrinogen (ACF). have diagnostic and prognostic value in early arthritis. Ann Rheum Dis. (2005) 64:1199–204. 10.1136/ard.2004.029389 - DOI - PMC - PubMed
-
- Horton SC, Tan AL, Wakefield RJ, Freeston JE, Buch MH, Emery P. Ultrasound-detectable grey scale synovitis predicts future fulfilment of the 2010 ACR/EULAR RA classification criteria in patients with new-onset undifferentiated arthritis. RMD Open (2017) 3:e000394. 10.1136/rmdopen-2016-000394 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

