Cyclophosphamide Pulse Therapy in Severe Refractory Crohn's Disease: A Retrospective Multicenter Case Series
- PMID: 30018965
- PMCID: PMC5988113
- DOI: 10.1159/000481820
Cyclophosphamide Pulse Therapy in Severe Refractory Crohn's Disease: A Retrospective Multicenter Case Series
Abstract
Background and aims: In Crohn's disease (CD) patients still remain refractory to current regimens, including biologicals. Previous data from small single-center studies indicated cyclophosphamide pulse therapy (CPT) to be effective for induction of remission at least in steroid-refractory cases. The aim of the present study was to study the efficacy and safety of CPT in mainly tumor necrosis factor (TNF)-refractory complicated CD patients.
Methods: Patients with refractory CD undergoing CPT were identified in 13 centers of the German IBD Study Group and retrospectively registered. In total, 41 patients (12 male, 29 female, median age 36 years, range 18-72 years) were included for analysis. Seventy-eight percent of these had previously been treated with thiopurines and 90% had previously received anti-TNF antibodies. Former steroid treatment was found throughout the cohort.
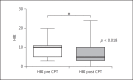
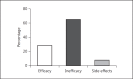
Results: Patients received a median number of 5 (1-13) pulses every 28 (13-54) days in a period of 120 (12-411) days. A median dose of 766 (600-1,200) mg and a median cumulative dose of 4,500 (750-9,750) mg was given. A clinical response (reduction in the Harvey-Bradshaw Index [HBI] ≥2 points) was found in 68% of the patients and clinical remission (HBI <5 points) in 32%. Steroids could be reduced from 31 to 12 mg per day over all patients. Side effects were recorded in 71% (n = 29) of the patients. Three patients terminated CPT due to side effects. No patient died.
Conclusion: Our data point to CPT as a therapeutic alternative for induction of remission in patients with severe refractory courses of CD including TNF antagonists. CPT might serve as bridging for maintenance treatment.
Keywords: Cyclophosphamide; Pulse therapy; Refractory Crohn's disease; Retrospective multicenter case series.
Figures

References
-
- Rungoe C, Langholz E, Andersson M, Basit S, Nielsen NM, Wohlfahrt J, Jess T. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut. 2014;63:1607–1616. - PubMed
-
- Xu P, He Y, Chen B, Mao R, Tang R, Li M, Chen F, Wu Y, Zeng Z, Chen M. Clinical characteristics and risk factors of refractory Crohn's disease. Zhonghua Yi Xue Za Zhi. 2014;94:2982–2987. - PubMed
-
- Hanauer SB. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: overview of randomized clinical studies. Rev Gastroenterol Disord. 2004;4((suppl 3)):S18–S24. - PubMed
-
- Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

