Clinical validation of the next-generation sequencing-based Extended RAS Panel assay using metastatic colorectal cancer patient samples from the phase 3 PRIME study
- PMID: 30019318
- PMCID: PMC6153611
- DOI: 10.1007/s00432-018-2688-3
Clinical validation of the next-generation sequencing-based Extended RAS Panel assay using metastatic colorectal cancer patient samples from the phase 3 PRIME study
Abstract
Purpose: To validate a next-generation sequencing (NGS)-based companion diagnostic using the MiSeqDx® sequencing instrument to simultaneously detect 56 RAS mutations in DNA extracted from formalin-fixed paraffin-embedded metastatic colorectal cancer (mCRC) tumor samples from the PRIME study. The test's ability to identify patients with mCRC likely to benefit from panitumumab treatment was assessed.
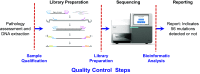
Methods: Samples from PRIME, which compared first-line panitumumab + FOLFOX4 with FOLFOX4, were processed according to predefined criteria using a multiplex assay that included input DNA qualification, library preparation, sequencing, and the bioinformatics reporting pipeline. NGS mutational analysis of KRAS and NRAS exons 2, 3, and 4 was performed and compared with Sanger sequencing.
Results: In 441 samples, positive percent agreement of the Extended RAS Panel with Sanger sequencing was 98.7% and negative percent agreement was 97.6%. For clinical validation (n = 528), progression-free survival (PFS) and overall survival (OS) were compared between patients with RAS mutations (RAS Positive) and those without (RAS Negative). Panitumumab + FOLFOX4 improved PFS in RAS Negative patients (P = 0.02). Quantitative interaction testing indicated the treatment effect (measured by the hazard ratio of panitumumab + FOLFOX4 versus FOLFOX4) differed for RAS Negative versus RAS Positive for PFS (P = 0.0038) and OS (P = 0.0323).
Conclusions: NGS allows for broad, rapid, highly specific analyses of genomic regions. These results support use of the Extended RAS Panel as a companion diagnostic for selecting patients for panitumumab, and utilization is consistent with recent clinical guidelines regarding mCRC RAS testing. Overall, approximately 13% more patients were detected with the Extended RAS Panel versus KRAS exon 2 alone.
Clinical trial registry identifier: NCT00364013 (ClinicalTrials.gov).
Keywords: Colorectal; Gastrointestinal cancers; Molecular diagnosis and prognosis; Mutation detection methods; New software for data analysis.
Conflict of interest statement
N. Udar and R. Slaughter are former employees of and own stock in Illumina, Inc. D. Vavrek, K. Meier, A. Iyer, and K. Gutekunst are employees of and own stock in Illumina, Inc. C. Lofton-Day, J. Dong (and spouse), and A. S. Jung are employees of and own stock in Amgen Inc. B. A. Bach is a former employee of and owns stock in Amgen Inc. M. Peeters has received research funding from Amgen Inc. and Roche, and has served on speakers bureaus for Amgen Inc., Sanofi, Servier, and Bayer. J.-Y. Douillard has nothing to disclose.
Figures


References
-
- Allegra CJ, Rumble RB, Hamilton SR, Mangu PB, Roach N, Hantel A, Schilsky RL (2016) Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology Provisional Clinical Opinion update 2015. J Clin Oncol 34:179–185 - PubMed
-
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634 - PubMed
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28:4697–4705 - PubMed
-
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD (2013) Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369:1023–1034 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

