Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease
- PMID: 30019767
- PMCID: PMC6513571
- DOI: 10.1002/14651858.CD012345.pub2
Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease
Update in
-
Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2018 Nov 27;11(11):CD012345. doi: 10.1002/14651858.CD012345.pub3. Cochrane Database Syst Rev. 2018. PMID: 30484282 Free PMC article.
Abstract
Background: Evidence on the health effects of total polyunsaturated fatty acids (PUFA) is equivocal. Fish oils are rich in omega-3 PUFA and plant oils in omega-6 PUFA. Evidence suggests that increasing PUFA-rich foods, supplements or supplemented foods can reduce serum cholesterol, but may increase body weight, so overall cardiovascular effects are unclear.
Objectives: To assess effects of increasing total PUFA intake on cardiovascular disease and all-cause mortality, lipids and adiposity in adults.
Search methods: We searched CENTRAL, MEDLINE and Embase to April 2017 and clinicaltrials.gov and the World Health Organization International Clinical Trials Registry Platform to September 2016, without language restrictions. We checked trials included in relevant systematic reviews.
Selection criteria: We included randomised controlled trials (RCTs) comparing higher with lower PUFA intakes in adults with or without cardiovascular disease that assessed effects over 12 months or longer. We included full texts, abstracts, trials registry entries and unpublished data. Outcomes were all-cause mortality, cardiovascular disease mortality and events, risk factors (blood lipids, adiposity, blood pressure), and adverse events. We excluded trials where we could not separate effects of PUFA intake from other dietary, lifestyle or medication interventions.
Data collection and analysis: Two review authors independently screened titles and abstracts, assessed trials for inclusion, extracted data, and assessed risk of bias. We wrote to authors of included trials for further data. Meta-analyses used random-effects analysis, sensitivity analyses included fixed-effects and limiting to low summary risk of bias. We assessed GRADE quality of evidence.
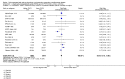
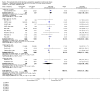
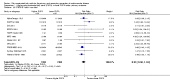
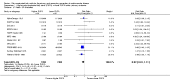
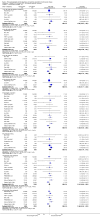
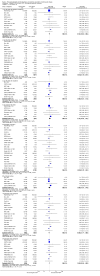
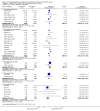
Main results: We included 49 RCTs randomising 24,272 participants, with duration of one to eight years. Eleven included trials were at low summary risk of bias, 33 recruited participants without cardiovascular disease. Baseline PUFA intake was unclear in most trials, but 3.9% to 8% of total energy intake where reported. Most trials gave supplemental capsules, but eight gave dietary advice, eight gave supplemental foods such as nuts or margarine, and three used a combination of methods to increase PUFA.Increasing PUFA intake probably has little or no effect on all-cause mortality (risk 7.8% vs 7.6%, risk ratio (RR) 0.98, 95% confidence interval (CI) 0.89 to 1.07, 19,290 participants in 24 trials), but probably slightly reduces risk of coronary heart disease events from 14.2% to 12.3% (RR 0.87, 95% CI 0.72 to 1.06, 15 trials, 10,076 participants) and cardiovascular disease events from 14.6% to 13.0% (RR 0.89, 95% CI 0.79 to 1.01, 17,799 participants in 21 trials), all moderate-quality evidence. Increasing PUFA may slightly reduce risk of coronary heart disease death (6.6% to 6.1%, RR 0.91, 95% CI 0.78 to 1.06, 9 trials, 8810 participants) andstroke (1.2% to 1.1%, RR 0.91, 95% CI 0.58 to 1.44, 11 trials, 14,742 participants, though confidence intervals include important harms), but has little or no effect on cardiovascular mortality (RR 1.02, 95% CI 0.82 to 1.26, 16 trials, 15,107 participants) all low-quality evidence. Effects of increasing PUFA on major adverse cardiac and cerebrovascular events and atrial fibrillation are unclear as evidence is of very low quality.Increasing PUFA intake slightly reduces total cholesterol (mean difference (MD) -0.12 mmol/L, 95% CI -0.23 to -0.02, 26 trials, 8072 participants) and probably slightly decreases triglycerides (MD -0.12 mmol/L, 95% CI -0.20 to -0.04, 20 trials, 3905 participants), but has little or no effect on high-density lipoprotein (HDL) (MD -0.01 mmol/L, 95% CI -0.02 to 0.01, 18 trials, 4674 participants) or low-density lipoprotein (LDL) (MD -0.01 mmol/L, 95% CI -0.09 to 0.06, 15 trials, 3362 participants). Increasing PUFA probably causes slight weight gain (MD 0.76 kg, 95% CI 0.34 to 1.19, 12 trials, 7100 participants).Effects of increasing PUFA on serious adverse events such as pulmonary embolism and bleeding are unclear as the evidence is of very low quality.
Authors' conclusions: This is the most extensive systematic review of RCTs conducted to date to assess effects of increasing PUFA on cardiovascular disease, mortality, lipids or adiposity. Increasing PUFA intake probably slightly reduces risk of coronary heart disease and cardiovascular disease events, may slightly reduce risk of coronary heart disease mortality and stroke (though not ruling out harms), but has little or no effect on all-cause or cardiovascular disease mortality. The mechanism may be via lipid reduction, but increasing PUFA probably slightly increases weight.
Conflict of interest statement
ASA: This review was funded by a grant from the World Health Organization. NM: None known CB: None known XW: This review was funded by a grant from the World Health Organization. JSB: This review was funded by a grant from the World Health Organization. TJB: This review was funded by a grant from the World Health Organization. SH: This review was funded by a grant from the World Health Organization. OFJ: This review was funded by a grant from the World Health Organization. SMAA: This review was funded by a grant from the World Health Organization. FS: This review was funded by a grant from the World Health Organization. KHOD: This review was funded by a grant from the World Health Organization. LH: This review was funded by a grant from the World Health Organization.
Figures

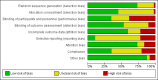

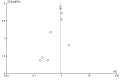

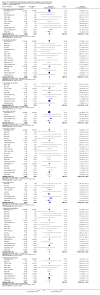


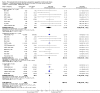

























































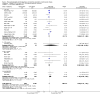















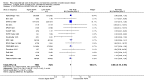


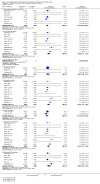










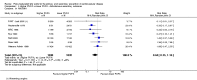

















































































References
References to studies included in this review
-
- Brouwer IA, Geleijnse JM, Klaasen VM, Smit LA, Giltay EJ, Goede J, et al. Effect of alpha linolenic acid supplementation on serum prostate specific antigen (PSA): results from the Alpha Omega Trial. PloS One 2013;8(12):e81519. - PMC - PubMed
- Eussen SR, Geleijnse JM, Giltay EJ, Rompelberg CJ, Klungel OH, Kromhout D. Effects of n‐3 fatty acids on major cardiovascular events in statin users and non‐users with a history of myocardial infarction. European Heart Journal 2012;33(13):1582‐8. - PMC - PubMed
- Geleijnse J, Giltay E, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2011;1:S512. - PubMed
- Geleijnse JM, Giltay EJ, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized, double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2012;8(4):278‐87. - PubMed
- Geleijnse JM, Giltay EJ, Schouten EG, Goede J, Oude Griep LM, Teitsma‐Jansen AM, et al. Effect of low doses of n‐3 fatty acids on cardiovascular diseases in 4,837 post‐myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. American Heart Journal 2010;159(4):539‐46. [DOI: 10/1016/j.ahj.2009.12.033] - PubMed
- Giltay EJ, Geleijnse JM, Heijboer AC, Goede J, Oude Griep LM, Blankenstein MA, et al. No effects of n‐3 fatty acid supplementation on serum total testosterone levels in older men: the Alpha Omega Trial. International Journal of Andrology 2012;35(5):680‐7. - PMC - PubMed
- Giltay EJ, Geleijnse JM, Kromhout D. Effects of n‐3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. American Journal of Clinical Nutrition 2011;94(6):1442‐50. - PMC - PubMed
- Hoogeveen E, Gemen E, Geleijnse M, Kusters R, Kromhout D, Giltay E. Effects of n‐3 fatty acids on decline of kidney function after myocardial infarction: Alpha Omega Trial. Nephrology, Dialysis, Transplantation 2012;27:ii64.
- Hoogeveen EK, Geleijnse JM, Kromhout D, Giltay EJ. No effect of n‐3 fatty acids on high‐sensitivity C‐reactive protein after myocardial infarction: the Alpha Omega Trial. European Journal of Preventive Cardiology 2014;21(11):1429‐36. - PubMed
- Hoogeveen EK, Geleijnse JM, Kromhout D, Stijnen T, Gemen EF, Kusters R, et al. Effect of omega‐3 fatty acids on kidney function after myocardial infarction: the Alpha Omega Trial. Clinical Journal of The American Society of Nephrology: CJASN 2014;9(10):1676‐83. - PMC - PubMed
- Kromhout D, Geleijnse JM, Goede J, Oude Griep LM, Mulder BJ, Boer MJ, et al. N‐3 fatty acids, ventricular arrhythmia‐related events, and fatal myocardial infarction in postmyocardial infarction patients with diabetes. Diabetes Care 2011;34(12):2515‐20. - PMC - PubMed
- Kromhout D, Giltay EJ, Geleijnse JM. N‐3 fatty acids and cardiovascular events after myocardial infarction. New England Journal of Medicine 2010;363(18):2015‐26. - PubMed
- NCT00127452. Alpha Omega Trial: study of omega‐3 fatty acids and coronary mortality. clinicaltrials.gov/ct2/show/NCT00127452 (first posted 5 August 2005).
-
- Bassey EJ, Littlewood JJ, Rothwell MC, Pye DW. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre‐ and postmenopausal women: two randomized controlled trials of Efacal v. calcium alone. British Journal of Nutrition 2000;83(6):629‐35. [PUBMED: 10911771] - PubMed
-
- Bassey EJ, Littlewood JJ, Rothwell MC, Pye DW. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre‐ and postmenopausal women: two randomized controlled trials of Efacal v. calcium alone. British Journal of Nutrition 2000;83(6):629‐35. [PUBMED: 10911771] - PubMed
-
- Bates D, Fawcett PR, Shaw DA, Weightman D. Trial of polyunsaturated fatty acids in non‐relapsing multiple sclerosis. British Medical Journal 1977;2(6092):932‐3. - PMC - PubMed
- Dworkin RH, Bates D, Millar JH, Paty DW. Linoleic acid and multiple sclerosis: a reanalysis of three double‐blind trials. Neurology 1984;34(11):1441‐5. - PubMed
References to studies excluded from this review
-
- Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. Journal of the American College of Cardiology 2014;64(14):1441‐8. - PubMed
- Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Multicentre trial of fish oil for the reduction of atrial fibrillation recurrence, inflammation and oxidative stress: the Atrial Fibrillation Fish Oil Research Study. Canadian Journal of Cardiology 2013;1:S383. - PubMed
-
- Brouwer IA, Geleijnse JM, Klaasen VM, Smit LA, Giltay EJ, Goede J, et al. Effect of alpha linolenic acid supplementation on serum prostate specific antigen (PSA): results from the alpha omega trial. PLoS One 2013;8(12):e81519. - PMC - PubMed
- Eussen SR, Geleijnse JM, Giltay EJ, Rompelberg CJ, Klungel OH, Kromhout D. Effects of n‐3 fatty acids on major cardiovascular events in statin users and non‐users with a history of myocardial infarction. European Heart Journal 2012;33(13):1582‐8. - PMC - PubMed
- Geleijnse J, Giltay E, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2011;1:S512. - PubMed
- Geleijnse JM, Giltay EJ, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized, double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2012;8(4):278‐87. - PubMed
- Geleijnse JM, Giltay EJ, Schouten EG, Goede J, Oude Griep LM, Teitsma‐Jansen AM, et al. Effect of low doses of n‐3 fatty acids on cardiovascular diseases in 4,837 post‐myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. American Heart Journal 2010;159(4):539‐46. [DOI: 10/1016/j.ahj.2009.12.033] - PubMed
- Giltay EJ, Geleijnse JM, Heijboer AC, Goede J, Oude Griep LM, Blankenstein MA, et al. No effects of n‐3 fatty acid supplementation on serum total testosterone levels in older men: the Alpha Omega Trial. International Journal of Andrology 2012;35(5):680‐7. - PMC - PubMed
- Giltay EJ, Geleijnse JM, Kromhout D. Effects of n‐3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. American Journal of Clinical Nutrition 2011;94(6):1442‐50. - PMC - PubMed
- Hoogeveen E, Gemen E, Geleijnse M, Kusters R, Kromhout D, Giltay E. Effects of n‐3 fatty acids on decline of kidney function after myocardial infarction: Alpha Omega trial. Nephrology Dialysis Transplantation 2012;27:ii64. - PMC - PubMed
- Hoogeveen EK, Geleijnse JM, Kromhout D, Giltay EJ. No effect of n‐3 fatty acids on high‐sensitivity C‐reactive protein after myocardial infarction: the Alpha Omega trial. European Journal of Preventive Cardiology 2014;21(11):1429‐36. - PubMed
- Hoogeveen EK, Geleijnse JM, Kromhout D, Stijnen T, Gemen EF, Kusters R, et al. Effect of omega‐3 fatty acids on kidney function after myocardial infarction: the Alpha Omega trial. Clinical Journal of The American Society of Nephrology: CJASN 2014;9(10):1676‐83. - PMC - PubMed
- Kromhout D, Geleijnse JM, Goede J, Oude Griep LM, Mulder BJ, Boer MJ, et al. N‐3 fatty acids, ventricular arrhythmia‐related events, and fatal myocardial infarction in postmyocardial infarction patients with diabetes. Diabetes Care 2011;34(12):2515‐20. - PMC - PubMed
- Kromhout D, Giltay EJ, Geleijnse JM. N‐3 fatty acids and cardiovascular events after myocardial infarction. New England Journal of Medicine 2010;363(18):2015‐26. - PubMed
-
- Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, et al. Effect of long‐chain omega‐3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age‐Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Internal Medicine 2014;174(5):763‐71. - PubMed
- Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, et al. The Age‐Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012;119(11):2282‐9. - PMC - PubMed
- Chew EY, Clemons TE. In reply: making sense of the evidence from the age‐related eye disease study 2 randomized clinical trial. JAMA Ophthalmology 2014;132(8):1031‐2. - PubMed
- Chew EY, Clemons TE, Agron E, Launer LJ, Grodstein F, Bernstein PS, et al. Effect of omega‐3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: the AREDS2 randomized clinical trial. JAMA 2015;314(8):791‐801. - PMC - PubMed
- Chew EY, Clemons TE, SanGiovanni JP, Danis RP, Ferris FL III, Elman MJ, et al. Secondary analyses of the effects of lutein/zeaxanthin on age‐related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmology 2014;132(2):142‐9. - PMC - PubMed
- Chew EY, SanGiovanni JP, Ferris FL, Wong WT, Agron E, Clemons TE, et al. Lutein/zeaxanthin for the treatment of age‐related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmology 2013;131(7):843‐50. - PMC - PubMed
- Huynh N, Nicholson BP, Agron E, Clemons TE, Bressler SB, Rosenfeld PJ, et al. Visual acuity after cataract surgery in patients with age‐related macular degeneration: age‐related eye disease study 2 report number 5. Ophthalmology 2014;121(6):1229‐36. - PMC - PubMed
-
- Bowman L, Aung T, Haynes R, Armitage J. ASCEND: design and baseline characteristics of a large randomised trial in diabetes. Diabetes 2012;61:A556‐7.
References to ongoing studies
-
- ACTRN12614000732684. The Aboriginal cardiovascular omega‐3 randomised controlled trial [The effect of omega‐3 supplementation on adverse cardiovascular (CV) events among Indigenous Australians with stable coronary artery disease: a randomized controlled trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366337 (date registered 10 July 2014).
-
- ACTRN12610000594022. Fish oil as adjunct therapy for periodontitis [Clinical efficacy of fish oil as adjunct therapy for patients with chronic periodontitis]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335470 (date registered 23 July 2010).
-
- ACTRN12613000034730. Intervention of testosterone & fish oil as a possible strategy for the prevention of Alzheimer’s Disease [A 56 week, double‐blind, randomised, placebo‐controlled trial to determine the efficacy of testosterone, with and without DHA supplementation in PiB positive men with subjective memory complaints as a strategy to prevent the development of Alzheimer’s disease]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363372 (date registered 14 January 2013).
-
- Holman RR, Paul S, Farmer A, Tucker L, Stratton IM, Neil HA, et al. Atorvastatin in factorial with omega‐3 EE90 risk reduction in diabetes (AFORRD): a randomised controlled trial. Diabetologia 2009;52(1):50‐9. - PubMed
- Neil HA, Ceglarek U, Thiery J, Paul S, Farmer A, Holman RR. Impact of atorvastatin and omega‐3 ethyl esters 90 on plasma plant sterol concentrations and cholesterol synthesis in type 2 diabetes: a randomised placebo controlled factorial trial. Atherosclerosis 2010;213(2):512‐7. - PubMed
-
- Cockayne NL, Duffy SL, Bonomally R, English A, Amminger PG, Mackinnon A, et al. The Beyond Ageing Project Phase 2‐‐a double‐blind, selective prevention, randomised, placebo‐controlled trial of omega‐3 fatty acids and sertraline in an older age cohort at risk for depression: study protocol for a randomized controlled trial. Trials 2015;16:247. - PMC - PubMed
Additional references
-
- 4S trial authors. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383‐9. - PubMed
-
- Abdelhamid A, Hooper L, Welch A. Polyunsaturated fatty acids for musculoskeletal health and functional status in older adults. PROSPERO2017; Vol. www.crd.york.ac.uk/prospero/display_record.php?RecordID=79211:CRD4201707....
-
- Berkley CS, Hoaglin DC, Mosteller F, Colditz GA. A random‐effects regression model for meta‐analysis. Statistics in Medicine 1995;14(4):395‐411. - PubMed
References to other published versions of this review
-
- Abdelhamid A, Martin N, Bridges C, Song F, Deane KHO, Hooper L. Polyunsaturated fat intake for prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012345] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

