Optimal site selection and image fusion guidance technology to facilitate cardiac resynchronization therapy
- PMID: 30019954
- PMCID: PMC6178093
- DOI: 10.1080/17434440.2018.1502084
Optimal site selection and image fusion guidance technology to facilitate cardiac resynchronization therapy
Abstract
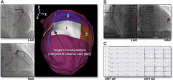
Introduction: Cardiac resynchronization therapy (CRT) has emerged as one of the few effective treatments for heart failure. However, up to 50% of patients derive no benefit. Suboptimal left ventricle (LV) lead position is a potential cause of poor outcomes while targeted lead deployment has been associated with enhanced response rates. Image-fusion guidance systems represent a novel approach to CRT delivery, allowing physicians to both accurately track and target a specific location during LV lead deployment.
Areas covered: This review will provide a comprehensive evaluation of how to define the optimal pacing site. We will evaluate the evidence for delivering targeted LV stimulation at sites displaying favorable viability or advantageous mechanical or electrical properties. Finally, we will evaluate several emerging image-fusion guidance systems which aim to facilitate optimal site selection during CRT.
Expert commentary: Targeted LV lead deployment is associated with reductions in morbidity and mortality. Assessment of tissue characterization and electrical latency are critical and can be achieved in a number of ways. Ultimately, the constraints of coronary sinus anatomy have forced the exploration of novel means of delivering CRT including endocardial pacing which hold promise for the future of CRT delivery.
Keywords: CRT; electrical latency; heart failure; image fusion; image guidance; mechanical latency; site selection; tissue viability.
Figures




References
-
- Petersen S, Rayner M, Wolstenholme J.. Coronary heart disease statistics: heart failure supplement. Br Hear Found. 2002.
-
- Cleland JGF, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. - PubMed
-
- Yu CM, Bleeker GB, Fung JW-H, et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–1586. - PubMed
-
- Mullens W, Grimm RA, Verga T, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765–773. - PubMed
-
- Kronborg MB, Johansen JB, Riahi S, et al. An anterior left ventricular lead position is associated with increased mortality and non-response in cardiac resynchronization therapy. Int J Cardiol. 2016;222:157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
