Patient-ventilator asynchrony
- PMID: 30020347
- PMCID: PMC6326703
- DOI: 10.1590/S1806-37562017000000185
Patient-ventilator asynchrony
Erratum in
-
ERRATUM.J Bras Pneumol. 2018 Jul-Aug;44(4):339. doi: 10.1590/S1806-37562017000000185errata. J Bras Pneumol. 2018. PMID: 30183973 Free PMC article.
Abstract
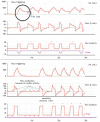
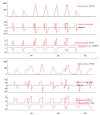
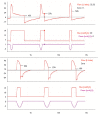
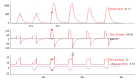
Patient-v entilator asynchrony (PVA) is a mismatch between the patient, regarding time, flow, volume, or pressure demands of the patient respiratory system, and the ventilator, which supplies such demands, during mechanical ventilation (MV). It is a common phenomenon, with incidence rates ranging from 10% to 85%. PVA might be due to factors related to the patient, to the ventilator, or both. The most common PVA types are those related to triggering, such as ineffective effort, auto-triggering, and double triggering; those related to premature or delayed cycling; and those related to insufficient or excessive flow. Each of these types can be detected by visual inspection of volume, flow, and pressure waveforms on the mechanical ventilator display. Specific ventilatory strategies can be used in combination with clinical management, such as controlling patient pain, anxiety, fever, etc. Deep sedation should be avoided whenever possible. PVA has been associated with unwanted outcomes, such as discomfort, dyspnea, worsening of pulmonary gas exchange, increased work of breathing, diaphragmatic injury, sleep impairment, and increased use of sedation or neuromuscular blockade, as well as increases in the duration of MV, weaning time, and mortality. Proportional assist ventilation and neurally adjusted ventilatory assist are modalities of partial ventilatory support that reduce PVA and have shown promise. This article reviews the literature on the types and causes of PVA, as well as the methods used in its evaluation, its potential implications in the recovery process of critically ill patients, and strategies for its resolution.
A assincronia pacie nte-ventilador (APV) é um desacoplamento entre o paciente, em relação a demandas de tempo, fluxo, volume e/ou pressão de seu sistema respiratório, e o ventilador, que as oferta durante a ventilação mecânica (VM). É um fenômeno comum, com taxas de incidência entre 10% e 85%. A APV pode ser devida a fatores relacionados ao paciente, ao ventilador ou a ambos. Os tipos de APV mais comuns são as de disparo, como esforço ineficaz; autodisparo e duplo disparo; as de ciclagem (tanto prematura quanto tardia); e as de fluxo (insuficiente ou excessivo). Cada um desses tipos pode ser detectado pela inspeção visual das curvas de volume-tempo, fluxo-tempo e pressãotempo na tela do ventilador mecânico. Estratégias ventilatórias específicas podem ser adotadas, em combinação com a abordagem clínica do paciente, como controle de dor, ansiedade, febre, etc. Níveis profundos de sedação devem ser evitados sempre que possível. A APV se associa a desfechos indesejados, tais como desconforto, dispneia, piora da troca gasosa, aumento do trabalho da respiração, lesão muscular diafragmática, prejuízo do sono, aumento da necessidade de sedação e/ou de bloqueio neuromuscular, assim como aumento do tempo de VM, de desmame e de mortalidade. A ventilação proporcional assistida e a ventilação assistida com ajuste neural são modalidades de suporte ventilatório parcial que reduzem a APV e têm se mostrado promissoras. Este artigo revisa a literatura acerca da APV abordando seus tipos, causas, métodos de avaliação, suas potenciais implicações no processo de recuperação de pacientes críticos e estratégias para sua resolução.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

