PCV13 vaccination impact: A multicenter study of pneumonia in 10 pediatric hospitals in Argentina
- PMID: 30020977
- PMCID: PMC6051625
- DOI: 10.1371/journal.pone.0199989
PCV13 vaccination impact: A multicenter study of pneumonia in 10 pediatric hospitals in Argentina
Abstract
Introduction: In 2012, PCV13 was introduced into the National Immunization Program in Argentina, 2+1 schedule for children <2 years. Coverage rates for 1st and 3rd doses were 69% and 41.0% in 2012, 98% and 86% in 2013; 99% and 89% in 2014, respectively. The aims of this study were to evaluate impact of PCV13 on Consolidated Pneumonia (CP) and Pneumococcal Pneumonia (PP) burden, and to describe epidemiological-clinical pattern of PP during the three-year period following vaccine introduction.
Methods: Hospital-based study at 10 pediatric surveillance units in Argentina. CP and PP discharge rates per 10,000 hospital discharges were compared between the pre-vaccination period 2007-2011 (preVp), the year of intervention (2012) and the post-vaccination period 2013-2014 (postVp).
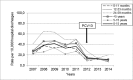
Results: Significant reduction in CP and PP discharge rates was observed in patients <5 years [% reduction (95%CI)]: 10.2% (6.3; 14.0) in 2012 and 24.8% (21.3; 28.2) in postVp for CP discharge rate; 59.5% (48.0; 68.5) in 2012 and 68.8% (58.3; 76.6) in postVp for PP discharge rate. Significant changes were also observed in children ≥5 years, mainly in PP discharge rate. A total of 297 PP cases were studied; 59.3% male; 31.3% <2 years; 42.9% had received PCV13 in 2012 and 84.5% in posVp. Case fatality rate was 3.4%. PCV13 serotypes decreased from 83.0% (39/47) in 2012 to 64.2% (52/81) in postVp, p = 0.039.
Conclusions: After PCV13 introduction, significant reduction in CP and PP discharge rates was observed in hospitalized children <5 years. In patients ≥5 years, PP discharge rate also decreased significantly.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Epidemiology of hospitalised paediatric community-acquired pneumonia and bacterial pneumonia following the introduction of 13-valent pneumococcal conjugate vaccine in the national immunisation programme in Japan.Epidemiol Infect. 2020 Apr 17;148:e91. doi: 10.1017/S0950268820000813. Epidemiol Infect. 2020. PMID: 32299523 Free PMC article.
-
Long-term Impact of a "3 + 0" Schedule for 7- and 13-Valent Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Australia, 2002-2014.Clin Infect Dis. 2017 Jan 15;64(2):175-183. doi: 10.1093/cid/ciw720. Epub 2016 Oct 21. Clin Infect Dis. 2017. PMID: 27986682
-
Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study.Lancet Infect Dis. 2015 May;15(5):535-43. doi: 10.1016/S1473-3099(15)70044-7. Epub 2015 Mar 20. Lancet Infect Dis. 2015. PMID: 25801458
-
Pneumococcal serotype evolution in Western Europe.BMC Infect Dis. 2015 Oct 14;15:419. doi: 10.1186/s12879-015-1147-x. BMC Infect Dis. 2015. PMID: 26468008 Free PMC article. Review.
-
The multifaceted impact of pneumococcal conjugate vaccine implementation in children in France between 2001 to 2014.Hum Vaccin Immunother. 2016;12(2):277-84. doi: 10.1080/21645515.2015.1116654. Hum Vaccin Immunother. 2016. PMID: 26905678 Free PMC article. Review.
Cited by
-
PCV20 for the prevention of invasive pneumococcal disease in the Mexican pediatric population: A cost-effectiveness analysis.Hum Vaccin Immunother. 2025 Dec;21(1):2475594. doi: 10.1080/21645515.2025.2475594. Epub 2025 Apr 3. Hum Vaccin Immunother. 2025. PMID: 40178501 Free PMC article.
-
Laboratory-based surveillance in Latin America: attributes and limitations in evaluation of pneumococcal vaccine impact.Hum Vaccin Immunother. 2021 Nov 2;17(11):4667-4672. doi: 10.1080/21645515.2021.1972709. Epub 2021 Oct 7. Hum Vaccin Immunother. 2021. PMID: 34618660 Free PMC article.
-
The direct effect of pneumococcal conjugate vaccines on invasive pneumococcal disease in children in the Latin American and Caribbean region (SIREVA 2006-17): a multicentre, retrospective observational study.Lancet Infect Dis. 2021 Mar;21(3):405-417. doi: 10.1016/S1473-3099(20)30489-8. Epub 2020 Sep 25. Lancet Infect Dis. 2021. PMID: 32986996 Free PMC article.
-
Invasive pneumococcal disease in Latin America and the Caribbean: Serotype distribution, disease burden, and impact of vaccination. A systematic review and meta-analysis.PLoS One. 2024 Jun 27;19(6):e0304978. doi: 10.1371/journal.pone.0304978. eCollection 2024. PLoS One. 2024. PMID: 38935748 Free PMC article.
-
Deep learning for classification of pediatric chest radiographs by WHO's standardized methodology.PLoS One. 2021 Jun 21;16(6):e0253239. doi: 10.1371/journal.pone.0253239. eCollection 2021. PLoS One. 2021. PMID: 34153076 Free PMC article.
References
-
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011; 66 (Suppl. 2):ii1–23. - PubMed
-
- World Health Organization. Pneumococcal vaccines WHO position paper—2012.Wkly Epidemiol Rec. 2012;87:129–144. - PubMed
-
- Rudan I, O’Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. Journal of Global Health. 2013;3(1):010401 10.7189/jogh.03.010401 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

