Ubiquitination of alpha-synuclein filaments by Nedd4 ligases
- PMID: 30021006
- PMCID: PMC6051637
- DOI: 10.1371/journal.pone.0200763
Ubiquitination of alpha-synuclein filaments by Nedd4 ligases
Erratum in
-
Correction: Ubiquitination of alpha-synuclein filaments by Nedd4 ligases.PLoS One. 2018 Aug 7;13(8):e0202158. doi: 10.1371/journal.pone.0202158. eCollection 2018. PLoS One. 2018. PMID: 30086168 Free PMC article.
Abstract
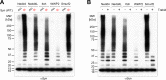
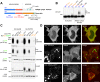
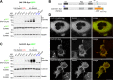
Alpha-synuclein can form beta-sheet filaments, the accumulation of which plays a key role in the development of Parkinson's disease, dementia with Lewy bodies and multiple system atrophy. It has previously been shown that alpha-synuclein is a substrate for the HECT domain-containing ubiquitin ligase Nedd4, and is subject to ubiquitin-mediated endosomal degradation. We show here that alpha-synuclein filaments are much better substrates for ubiquitination in vitro than monomeric alpha-synuclein, and that this increased susceptibility cannot be mimicked by the mere clustering of monomers. Recognition by Nedd4 family enzymes is not through the conventional binding of PPxY-containing sequences to WW domains of the ligase, but it also involves C2 and HECT domains. The disease-causing alpha-synuclein mutant A53T is a much less efficient substrate for Nedd4 ligases than the wild-type protein. We suggest that preferential recognition, ubiquitination and degradation of beta-sheet-containing filaments may help to limit toxicity, and that A53T alpha-synuclein may be more toxic, at least in part because it avoids this fate.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

