A Novel Squirrel Respirovirus with Putative Zoonotic Potential
- PMID: 30021939
- PMCID: PMC6070802
- DOI: 10.3390/v10070373
A Novel Squirrel Respirovirus with Putative Zoonotic Potential
Abstract
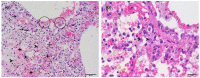
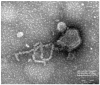
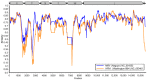
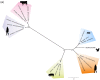
In a globalized world, the threat of emerging pathogens plays an increasing role, especially if their zoonotic potential is unknown. In this study, a novel respirovirus, family Paramyxoviridae, was isolated from a Sri Lankan Giant squirrel (Ratufa macroura), which originated in Sri Lanka and deceased with severe pneumonia in a German zoo. The full-genome characterization of this novel virus, tentatively named Giant squirrel respirovirus (GSqRV), revealed similarities to murine (71%), as well as human respiroviruses (68%) with unique features, for example, a different genome length and a putative additional accessory protein. Congruently, phylogenetic analyses showed a solitary position of GSqRV between known murine and human respiroviruses, implicating a putative zoonotic potential. A tailored real-time reverse transcription-polymerase chain reaction (RT-qPCR) for specific detection of GSqRV confirmed a very high viral load in the lung, and, to a lesser extent, in the brain of the deceased animal. A pilot study on indigenous and exotic squirrels did not reveal additional cases in Germany. Therefore, further research is essential to assess the geographic distribution, host range, and zoonotic potential of this novel viral pathogen.
Keywords: Paramyxoviridae; Sri Lankan Giant squirrel; isolate; novel respirovirus; pneumonia; potential zoonosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Introduction and spread of variegated squirrel bornavirus 1 (VSBV-1) between exotic squirrels and spill-over infections to humans in Germany.Emerg Microbes Infect. 2021 Dec;10(1):602-611. doi: 10.1080/22221751.2021.1902752. Emerg Microbes Infect. 2021. PMID: 33706665 Free PMC article.
-
Assessing the occurrence of the novel zoonotic variegated squirrel bornavirus 1 in captive squirrels in Germany -A prevalence study.Zoonoses Public Health. 2021 Mar;68(2):110-120. doi: 10.1111/zph.12801. Epub 2021 Jan 11. Zoonoses Public Health. 2021. PMID: 33428333
-
Tioman virus, a novel paramyxovirus isolated from fruit bats in Malaysia.Virology. 2001 May 10;283(2):215-29. doi: 10.1006/viro.2000.0882. Virology. 2001. PMID: 11336547
-
Respiratory virus infection in infants and children.Pediatr Dev Pathol. 2007 May-Jun;10(3):172-80. doi: 10.2350/07-02-0238.1. Pediatr Dev Pathol. 2007. PMID: 17535096 Review.
-
The replicative complex of paramyxoviruses: structure and function.Adv Virus Res. 1998;50:101-39. doi: 10.1016/s0065-3527(08)60807-6. Adv Virus Res. 1998. PMID: 9520998 Review. No abstract available.
Cited by
-
Discovery and characterization of novel paramyxoviruses from bat samples in China.Virol Sin. 2023 Apr;38(2):198-207. doi: 10.1016/j.virs.2023.01.002. Epub 2023 Jan 14. Virol Sin. 2023. PMID: 36649817 Free PMC article.
-
Identification and Genetic Characterization of a Novel Respirovirus in Alpine Chamois (rupicapra rupicapra rupicapra).Animals (Basel). 2020 Apr 17;10(4):704. doi: 10.3390/ani10040704. Animals (Basel). 2020. PMID: 32316522 Free PMC article.
-
Divergent Viruses Discovered in Swine Alter the Understanding of Evolutionary History and Genetic Diversity of the Respirovirus Genus and Related Porcine Parainfluenza Viruses.Microbiol Spectr. 2022 Jun 29;10(3):e0024222. doi: 10.1128/spectrum.00242-22. Epub 2022 Jun 1. Microbiol Spectr. 2022. PMID: 35647875 Free PMC article.
-
Next-generation diagnostics: virus capture facilitates a sensitive viral diagnosis for epizootic and zoonotic pathogens including SARS-CoV-2.Microbiome. 2021 Feb 20;9(1):51. doi: 10.1186/s40168-020-00973-z. Microbiome. 2021. PMID: 33610182 Free PMC article.
-
Taxonomic Changes for Human and Animal Viruses, 2016 to 2018.J Clin Microbiol. 2019 Jan 30;57(2):e01457-18. doi: 10.1128/JCM.01457-18. Print 2019 Feb. J Clin Microbiol. 2019. PMID: 30305385 Free PMC article. Review.
References
-
- Wang L.-F., Chua K., Yu M., Eaton B.T. Genome Diversity of Emerging Paramyxoviruses. Curr. Genom. 2003;4:263–273. doi: 10.2174/1389202033490385. - DOI
-
- Lau S.K.P., Woo P.C.Y., Wong B.H.L., Wong A.Y.P., Tsoi H.-W., Wang M., Lee P., Xu H., Poon R.W.S., Guo R., et al. Identification and complete genome analysis of three novel paramyxoviruses, Tuhoko virus 1, 2 and 3, in fruit bats from China. Virology. 2010;404:106–116. doi: 10.1016/j.virol.2010.03.049. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

