Quantitative muscle MRI to follow up late onset Pompe patients: a prospective study
- PMID: 30022036
- PMCID: PMC6052002
- DOI: 10.1038/s41598-018-29170-7
Quantitative muscle MRI to follow up late onset Pompe patients: a prospective study
Abstract
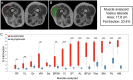
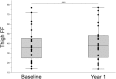
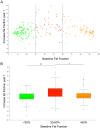
Late onset Pompe disease (LOPD) is a slow, progressive disorder characterized by skeletal and respiratory muscle weakness. Enzyme replacement therapy (ERT) slows down the progression of muscle symptoms. Reliable biomarkers are needed to follow up ERT-treated and asymptomatic LOPD patients in clinical practice. In this study, 32 LOPD patients (22 symptomatic and 10 asymptomatic) underwent muscle MRI using 3-point Dixon and were evaluated at the time of the MRI with several motor function tests and patient-reported outcome measures, and again after one year. Muscle MRI showed a significant increase of 1.7% in the fat content of the thigh muscles in symptomatic LOPD patients. In contrast, there were no noteworthy differences between muscle function tests in the same period of time. We did not observe any significant changes either in muscle MRI or in muscle function tests in asymptomatic patients over the year. We conclude that 3-point Dixon muscle MRI is a useful tool for detecting changes in muscle structure in symptomatic LOPD patients and could become part of the current follow-up protocol in daily clinics.
Conflict of interest statement
The study was supported by Sanofi-Genzyme. The company did not interfere in the design of the protocol or review any data obtained from patients. There are no other conflicts of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

