p38-mediated phosphorylation at T367 induces EZH2 cytoplasmic localization to promote breast cancer metastasis
- PMID: 30022044
- PMCID: PMC6051995
- DOI: 10.1038/s41467-018-05078-8
p38-mediated phosphorylation at T367 induces EZH2 cytoplasmic localization to promote breast cancer metastasis
Abstract
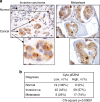
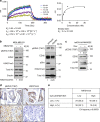
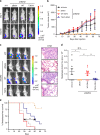
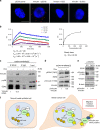
Overexpression of EZH2 in estrogen receptor negative (ER-) breast cancer promotes metastasis. EZH2 has been mainly studied as the catalytic component of the Polycomb Repressive Complex 2 (PRC2) that mediates gene repression by trimethylating histone H3 at lysine 27 (H3K27me3). However, how EZH2 drives metastasis despite the low H3K27me3 levels observed in ER- breast cancer is unknown. Here we show that in human invasive carcinomas and distant metastases, cytoplasmic EZH2 phosphorylated at T367 is significantly associated with ER- disease and low H3K27me3 levels. p38-mediated EZH2 phosphorylation at T367 promotes EZH2 cytoplasmic localization and potentiates EZH2 binding to vinculin and other cytoskeletal regulators of cell migration and invasion. Ectopic expression of a phospho-deficient T367A-EZH2 mutant is sufficient to inhibit EZH2 cytoplasmic expression, disrupt binding to cytoskeletal regulators, and reduce EZH2-mediated adhesion, migration, invasion, and development of spontaneous metastasis. These results point to a PRC2-independent non-canonical mechanism of EZH2 pro-metastatic function.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Howlader N, et al. (eds). SEER Cancer Statistics Review, 1975–2013 (National Cancer Institute, Bethesda, MD, 2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

