The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding
- PMID: 30022052
- PMCID: PMC6052114
- DOI: 10.1038/s41467-018-05253-x
The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding
Erratum in
-
Author Correction: The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding.Nat Commun. 2020 Aug 19;11(1):4210. doi: 10.1038/s41467-020-18023-5. Nat Commun. 2020. PMID: 32814768 Free PMC article.
Abstract
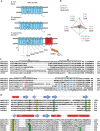
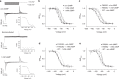
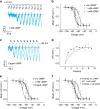
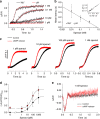
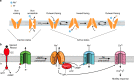
Voltage-sensing (VSD) and cyclic nucleotide-binding domains (CNBD) gate ion channels for rapid electrical signaling. By contrast, solute carriers (SLCs) that passively redistribute substrates are gated by their substrates themselves. Here, we study the orphan sperm-specific solute carriers SLC9C1 that feature a unique tripartite structure: an exchanger domain, a VSD, and a CNBD. Voltage-clamp fluorimetry shows that SLC9C1 is a genuine Na+/H+ exchanger gated by voltage. The cellular messenger cAMP shifts the voltage range of activation. Mutations in the transport domain, the VSD, or the CNBD strongly affect Na+/H+ exchange, voltage gating, or cAMP sensitivity, respectively. Our results establish SLC9C1 as a phylogenetic chimaera that combines the ion-exchange mechanism of solute carriers with the gating mechanism of ion channels. Classic SLCs slowly readjust changes in the intra- and extracellular milieu, whereas voltage gating endows the Na+/H+ exchanger with the ability to produce a rapid pH response that enables downstream signaling events.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

