Bistable and photoswitchable states of matter
- PMID: 30022053
- PMCID: PMC6052001
- DOI: 10.1038/s41467-018-05300-7
Bistable and photoswitchable states of matter
Erratum in
-
Publisher Correction: Bistable and photoswitchable states of matter.Nat Commun. 2018 Aug 7;9(1):3204. doi: 10.1038/s41467-018-05789-y. Nat Commun. 2018. PMID: 30087353 Free PMC article.
Abstract
Classical materials readily switch phases (solid to fluid or fluid to gas) upon changes in pressure or heat; however, subsequent reversion of the stimulus returns the material to their original phase. Covalently cross-linked polymer networks, which are solids that do not flow when strained, do not change phase even upon changes in temperature and pressure. However, upon the addition of dynamic cross-links, they become stimuli responsive, capable of switching phase from solid to fluid, but quickly returning to the solid state once the stimulus is removed. Reported here is the first material capable of a bistable switching of phase. A permanent solid to fluid transition or vice versa is demonstrated at room temperature, with inherent, spatiotemporal control over this switch in either direction triggered by exposure to light.
Conflict of interest statement
The authors declare no competing interests.
Figures
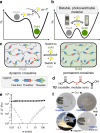
 black filled circle; G″ =
black filled circle; G″ =  black open circle), and as solids when the base catalyst is removed (G′ =
black open circle), and as solids when the base catalyst is removed (G′ =  grey filled square; G″ =
grey filled square; G″ =  grey open square). e The ability of thioester crosslinked materials while in the fluid phase to undergo large changes in structure at room temperature is indicated by the ability to shred and subsequently heal the material into a defect free, optically active material
grey open square). e The ability of thioester crosslinked materials while in the fluid phase to undergo large changes in structure at room temperature is indicated by the ability to shred and subsequently heal the material into a defect free, optically active material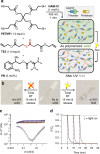
 grey filled square; G″ =
grey filled square; G″ =  grey open square; 1.5 h after polymerization: G′ =
grey open square; 1.5 h after polymerization: G′ =  red filled circle; G″ =
red filled circle; G″ =  red open circle) until exposure to UV light, which switches it to a fluid (immediately after UV light: G′ =
red open circle) until exposure to UV light, which switches it to a fluid (immediately after UV light: G′ =  black filled triangle; G″ =
black filled triangle; G″ =  black open triangle; 1.5 h after UV light: G′ =
black open triangle; 1.5 h after UV light: G′ =  blue filled diamond; G″ =
blue filled diamond; G″ =  blue open diamond). d The nearly instantaneous fluidization of the network upon exposure to UV light, shown here by the relaxation of stress at a constant strain (10% strain, light on at 5 (blue), 10 (red), and 15 (green) minutes, continuously irradiated, 365 nm, 75 mW/cm2). Grey line—not irradiated
blue open diamond). d The nearly instantaneous fluidization of the network upon exposure to UV light, shown here by the relaxation of stress at a constant strain (10% strain, light on at 5 (blue), 10 (red), and 15 (green) minutes, continuously irradiated, 365 nm, 75 mW/cm2). Grey line—not irradiated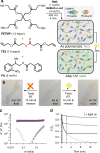
 grey filled square; G″ =
grey filled square; G″ =  grey open square; 1.5 h after polymerization: G′ =
grey open square; 1.5 h after polymerization: G′ =  red filled circle; G″ =
red filled circle; G″ =  red open circle) until exposure to UV light, which switches it to a solid (immediately after irradiation: G′ =
red open circle) until exposure to UV light, which switches it to a solid (immediately after irradiation: G′ =  black filled triangle; G″ =
black filled triangle; G″ =  black open triangle; 1.5 h after irradiation: G′ =
black open triangle; 1.5 h after irradiation: G′ =  blue filled diamond; G″ =
blue filled diamond; G″ =  blue open diamond). d The nearly instantaneous solidification of the material upon exposure to UV light, shown here by the relaxation of stress at a constant strain (10% strain, light on at 5 (blue), 20 (red), and 120 (black) seconds, irradiated for 120 s, 320–500 nm, 75 mW/cm2, a small thermal recovery was noted in each case after the light was turned off). Grey line—not irradiated
blue open diamond). d The nearly instantaneous solidification of the material upon exposure to UV light, shown here by the relaxation of stress at a constant strain (10% strain, light on at 5 (blue), 20 (red), and 120 (black) seconds, irradiated for 120 s, 320–500 nm, 75 mW/cm2, a small thermal recovery was noted in each case after the light was turned off). Grey line—not irradiated
References
-
- Goodstein, D. L. States of Matter. (Dover Publications, Inc, Mineola, NY, 2014).
-
- Odian, G. G. Principles of Polymerization. (John Wiley & Sons, NJ, 2004).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

