Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability
- PMID: 30022154
- PMCID: PMC6786410
- DOI: 10.1038/s41401-018-0045-3
Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability
Abstract
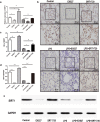
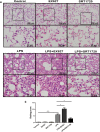
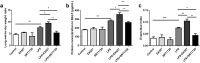
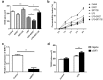
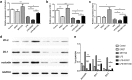
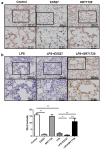
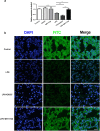
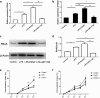
The integrity of the endothelial barrier is a determinant of the prognosis of lipopolysaccharide (LPS)-induced acute lung injury (ALI). In this study, we investigated whether and how Sirtuin 1 (SIRT1) maintained the vascular integrity during ALI. An experimental model of ALI was established in mice through intratracheal administration of LPS (10 mg/kg). LPS stimulation significantly increased the pulmonary permeability and decreased the expression of SIRT1 and tight junction proteins (TJs), including occludin, claudin-5, tight junction protein 1 and tight junction protein 2. Morphological studies showed that LPS induced obvious lung injury with inflammatory cell infiltration in the interstitial and alveolar space, hemorrhage, edema, and the thickened alveolar wall compared to the control mice. Intratracheal administration of the selective SIRT1 activator SRT1720 (6.25 mg/kg) significantly attenuated LPS-induced lung injury, lung hyper-permeability and increased TJs expression, whereas intratracheal administration of the selective SIRT1 inhibitor EX527 (6.25 mg/kg) aggravated LPS-induced ALI. Similar protective effects of SIRT1 on pulmonary cellular permeability were observed in primary human pulmonary microvascular endothelial cells treated with LPS (2 mg/mL) in vitro. We further demonstrated that the RhoA/ROCK signaling pathway was activated in SIRT1 regulation of tight junction permeability. The RhoA/ROCK inhibitor Y-27632 (10 μM) increased the expression of TJs and reversed LPS- or EX527-induced hyper-permeability. In conclusion, SIRT1 ameliorates LPS-induced lung injury via decreasing endothelial tight junction permeability, possibly via RhoA/ROCK signaling pathway. This finding may contribute to the development of new therapeutic approaches for lung injury.
Keywords: EX527; LPS; RhoA/ROCK; SIRT1 protein; SRT1720; Y-27632; capillary endothelial permeability; human pulmonary microvascular endothelial cells; lung injury; tight junction proteins.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

