The outer membrane is an essential load-bearing element in Gram-negative bacteria
- PMID: 30022160
- PMCID: PMC6089221
- DOI: 10.1038/s41586-018-0344-3
The outer membrane is an essential load-bearing element in Gram-negative bacteria
Abstract
Gram-negative bacteria possess a complex cell envelope that consists of a plasma membrane, a peptidoglycan cell wall and an outer membrane. The envelope is a selective chemical barrier1 that defines cell shape2 and allows the cell to sustain large mechanical loads such as turgor pressure3. It is widely believed that the covalently cross-linked cell wall underpins the mechanical properties of the envelope4,5. Here we show that the stiffness and strength of Escherichia coli cells are largely due to the outer membrane. Compromising the outer membrane, either chemically or genetically, greatly increased deformation of the cell envelope in response to stretching, bending and indentation forces, and induced increased levels of cell lysis upon mechanical perturbation and during L-form proliferation. Both lipopolysaccharides and proteins contributed to the stiffness of the outer membrane. These findings overturn the prevailing dogma that the cell wall is the dominant mechanical element within Gram-negative bacteria, instead demonstrating that the outer membrane can be stiffer than the cell wall, and that mechanical loads are often balanced between these structures.
Conflict of interest statement
The authors have no competing financial interests.
Figures




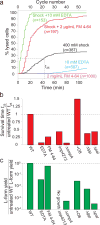
Comment in
-
Microbiology: Peeling Back the Layers of Bacterial Envelope Mechanics.Curr Biol. 2018 Oct 22;28(20):R1210-R1211. doi: 10.1016/j.cub.2018.09.023. Curr Biol. 2018. PMID: 30352194
References
-
- Martin H, Frank H. Quantitative Bausteinanalyse der Stützmembran in der Zellwand von Escherichia coli B. Zeitschrift für Naturforschung B. 1962;17:190–196.
-
- Deng Y, Sun M, Shaevitz JW. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys Rev Lett. 2011;107:158101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

