Bilateral versus unilateral botulinum toxin injections for chronic anal fissure: a randomised trial
- PMID: 30022331
- PMCID: PMC6097731
- DOI: 10.1007/s10151-018-1821-2
Bilateral versus unilateral botulinum toxin injections for chronic anal fissure: a randomised trial
Abstract
Background: Botulinum toxin injected into the internal anal sphincter is used in the treatment of chronic anal fissure but there is no standardised technique for its administration. This randomised single centre trial compares bilateral (either side of fissure) to unilateral injection.
Methods: Participants were randomised to receive bilateral (50 + 50 units) or unilateral (100 units) Dysport® injections into the internal anal sphincter in an outpatient setting. Injection-related pain assessed by visual analogue scale was the primary outcome measure. Secondary outcomes were healing rate, fissure pain, incontinence, and global health scores.
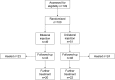
Results: Between October 2008 and April 2012, 100 patients with chronic anal fissure were randomised to receive bilateral or unilateral injections. Injection-related pain was comparable in both groups. There was no difference in healing rate. Initially, there was greater improvement in fissure pain in the bilateral group but at 1 year the unilateral group showed greater improvement. Cleveland Clinic Incontinence score was lower in the unilateral group in the early post-treatment period and global health assessment (EuroQol EQ-VAS) was higher in the unilateral group at 1 year.
Conclusions: Injection-related pain was similar in bilateral and unilateral injection groups. Unilateral injection was as effective as bilateral injections in healing and improving fissure pain without any deterioration in continence.
Keywords: Anal fistula; Botulinum toxin; Injection.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous