Risperidone for children and adolescents with autism spectrum disorder: a systematic review
- PMID: 30022830
- PMCID: PMC6045903
- DOI: 10.2147/NDT.S151802
Risperidone for children and adolescents with autism spectrum disorder: a systematic review
Abstract
Background: Various clinical trials suggested that risperidone was beneficial in the treatment of autism spectrum disorder (ASD) in children and adolescents.
Objective: The aim of this systematic review was to determine the efficacy, acceptability and tolerability of risperidone in the treatment of children and adolescents with ASD.
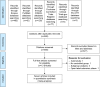
Data sources: The databases of Scopus, PubMed, CINAHL and Cochrane Controlled Trials Register were searched in February 2017.
Study eligibility criteria participants and interventions: Eligible RCTs of risperidone in the treatment of child and adolescent patients with ASD. Languages were not restricted.
Study appraisal and synthesis methods: The full-text versions of relevant studies were thoroughly assessed and extracted. The primary efficacy of outcome was the pooled response rate and the pooled mean changed scores of the standardized rating scales for ASD.
Results: A total of 372 randomized subjects from seven RCTs were included in this review. In acute treatment, the pooled mean change score of the Aberrant Behavior Checklist for irritability subscale (ABC-I) and response rate for the risperidone-treated group had a greater significance than that of the placebo-treated group. In the long-term treatment, the pooled mean change score of the CARS in the risperidone-treated group was significantly greater than that in the placebo-treated group. According to the discontinuation phase, the overall pooled relapse rate of the risperidone-treated group was significantly less than that of the placebo-treated group. The rates of pooled overall discontinuation and discontinuation due to adverse events rates were not different between the two groups in acute and long-term treatments.
Limitations: A small study was included in the current review.
Conclusion: In relation to the current systematic review, risperidone is efficacious in the treatment of symptoms in children and adolescents with ASD. Although its acceptability is comparable to placebo, treatment with risperidone is well tolerated in children and adolescents with ASD.
Keywords: ABC; Aberrant Behavior Checklist; CARS; Childhood Autism Rating Scale; acceptability; efficacy; tolerability.
Conflict of interest statement
Disclosure NM received travel reimbursement from Lundbeck and Pfizer. BM received honoraria and/or travel reimbursement from Lundbeck, Pfizer and Servier. MS received honoraria from Lundbeck and Sumitomo Dainippon Pharma. The authors report no other conflicts of interest in this work.
Figures


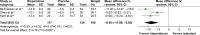

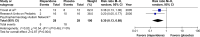
References
-
- Schreibman L. Intensive behavioral/psychoeducational treatments for autism: research needs and future directions. J Autism Dev Disord. 2000;30(5):373–378. - PubMed
-
- Spreckley M, Boyd R. Efficacy of applied behavioral intervention in preschool children with autism for improving cognitive, language, and adaptive behavior: a systematic review and meta-analysis. J Pediatr. 2009;154(3):338–344. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

