A novel animal model for residence time evaluation of injectable hyaluronic acid-based fillers using high-frequency ultrasound-based approach
- PMID: 30022845
- PMCID: PMC6045909
- DOI: 10.2147/CCID.S156740
A novel animal model for residence time evaluation of injectable hyaluronic acid-based fillers using high-frequency ultrasound-based approach
Abstract
Background: Hyaluronic acid (HA)-based devices are among the most popular filler agents for skin rejuvenation. One of the principal goals is the improvement in residence time of HA-based products, to increase their performance and reduce frequency of the treatment. So, understanding fillers, behavior after subcutaneous injection is a fundamental aspect for discovery and optimization of new products. Current in vivo approaches to detect/quantify injected HA fillers are not always well optimized or easy to apply.
Objective: To develop more efficacious and noninvasive diagnostic tools to make a quantitative evaluation of the degradation of fillers in a small animal model.
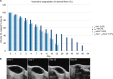
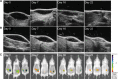
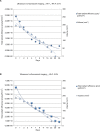
Materials and methods: We evaluated the residence time of different HA-based fillers, fluorescein-labeled and not, injected subcutaneously in mice. Volumes of fillers were monitored through high-frequency ultrasound (HF-US) method while fluorescence intensity through the well-established fluorescence living imaging method. To confirm the effectiveness of HF-US, obtained volumetric measurements were compared with fluorescence intensity values.
Results: Both the presented methods revealed the same degradation kinetics for the tested products.
Conclusion: The two used methods are fully comparable and quantitatively accurate. The presented approach has been proved to be noninvasive, sensitive, and reproducible.
Keywords: HF-US; animal model; dermal filler; hyaluronan; hyaluronic acid; ultrasound.
Conflict of interest statement
Disclosure Andrea Maria Giori is an employee of IBSA Farmaceutici Italia, a pharmaceutical company based in Lodi, Italy. Filomena Merola, Mario Scrima, Carmela Melito, Antonio Iorio, and Angela Ferravante are employees of Bouty SpA., a pharmaceutical company based in Milan, Italy. Claudio Pisano is a consultant of Bouty SpA. The authors report no other conflicts of interest in this work.
Figures

References
-
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. - PubMed
-
- Urbaniak B, Plewa S, Zenon J, Kokot Z. Preliminary high performance capillary electrophoresis (HPCE) studies of enzymatic degradation of hyaluronic acid by hyaluronidase in the presence of polyvalent metal ions. Acta Pol Pharm. 2017;74:41–51. - PubMed
-
- Buck DW, Alam M, Kim JY. Injectable fillers for facial rejuvenation: a review. J Plast ReconstrAesthet Surg. 2009;62:11–18. - PubMed
-
- Namiki O, Toyoshima H, Morisaki N. Therapeutic effect of intra-articular injection of high molecular weight hyaluronic acid on osteoarthritis of the knee. Int J Clin Pharmacol Ther Toxicol. 1982;20:501–507. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

