Risk analysis and assessment based on Sigma metrics and intended use
- PMID: 30022882
- PMCID: PMC6039164
- DOI: 10.11613/BM.2018.020707
Risk analysis and assessment based on Sigma metrics and intended use
Abstract
Introduction: In order to ensure the quality in clinical laboratories and meet the low risk requirements of patients and clinicians, a risk analysis and assessment model based on Sigma metrics and intended use was constructed, based on which differential sigma performance (σ) expectations of 42 analytes were developed.
Materials and methods: Failure mode and effects analysis was applied to produce an analytic risk rating based on three factors, each test of which was graded as follows: 1) Sigma metrics; 2) the severity of harm; 3) intended use. By multiplying the score of Sigma metrics by the score of severity of harm by the score of intended use, each was assigned a typical risk priority number (RPN), with RPN ≤ 25 rated as low risk. Low risk was defined as acceptable standards; the sigma performance expectations were calculated.
Results: Among the 42 analytes, tests with σ ≥ 6, 5 ≤ σ < 6, 4 ≤ σ < 5, 3 ≤ σ < 4, σ < 3 were 21, 5, 5, 6, and 5, respectively; there were 7 high-risk tests, 8 of them medium risk tests. According to the risk assessment conclusion, 13 tests had sigma performance expectations ≥ 6; 15 test items had sigma performance expectations ≥ 5, while 3 test items had sigma performance expectations ≥ 4; 11 test items had sigma performance expectations ≥ 3.
Conclusions: Constructing the risk analysis and assessment model based on Sigma metrics and intended use will help clinical laboratories to identify the high-risk tests more objectively and comprehensively. Such model can also be used to establish the sigma performance expectations and meet the low risk requirements of patients and clinicians.
Keywords: Sigma metrics; Six Sigma; failure mode and effects analysis; risk analysis; risk assessment.
Conflict of interest statement
Potential conflict of interest: None declared.
Figures
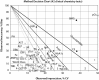
References
-
- Clinical and Laboratory Standards Institute (CLSI). Laboratory quality control based on risk management - Approved Guideline. CLSI document EP23-A. Wayne: CLSI, 2011.
-
- International Organization for Standardization (ISO). ISO 15189:2012. Medical laboratories – requirements for quality and competence. Geneva: ISO, 2012.
-
- Clinical and Laboratory Standards Institute (CLSI). Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions - Approved Guideline. CLSI document C24. Wayne: CLSI, 2016.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

