Expression and (Lacking) Internalization of the Cell Surface Receptors of Clostridioides difficile Toxin B
- PMID: 30022975
- PMCID: PMC6039548
- DOI: 10.3389/fmicb.2018.01483
Expression and (Lacking) Internalization of the Cell Surface Receptors of Clostridioides difficile Toxin B
Abstract
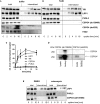
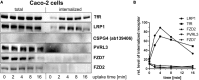
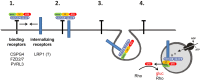
Toxin-producing strains of Clostridioides difficile and Clostridium perfringens cause infections of the gastrointestinal tract in humans and ruminants, with the toxins being major virulence factors, essential for the infection, and responsible for the onset of severe symptoms. C. difficile toxin A (TcdA) and toxin B (TcdB), and the large cytotoxin (TpeL) from C. perfringens are single chain bacterial protein toxins with an AB-like toxin structure. The C-terminal delivery domain mediates cell entry of the N-terminal glycosyltransferase domain by receptor-mediated endocytosis. Several cell surface proteins have been proposed to serve as toxin receptors, including chondroitin-sulfate proteoglycan 4 (CSPG4), poliovirus receptor-like 3 (PVRL3), and frizzled-1/2/7 (FZD1/2/7) for TcdB and LDL-receptor-related protein-1 (LRP1) for TpeL. The expression of the TcdB receptors was investigated in human intestinal organoids (HIOs) and in cultured cell lines. HIOs from four human donors exhibited a comparable profile of receptor expression, with PVRL3, LRP1, and FZD7 being expressed and CSPG4 and FZD2 not being expressed. In human epithelial Caco-2 cells and HT29 cells as well as in immortalized murine fibroblasts, either receptor FZD2/7, CSPG4, PVRL3, and LRP1 was expressed. The question whether the toxins take advantage of the normal turnover of their receptors (i.e., constitutive endocytosis and recycling) from the cell surface or whether the toxins activity induce the internalization of their receptors has not yet been addressed. For the analysis of receptor internalization, temperature-induced uptake of biotinylated toxin receptors into immortalized mouse embryonic fibroblasts (MEFs) and Caco-2 cells was exploited. Solely LRP1 exhibited constitutive endocytosis from the plasma membrane to the endosome, which might be abused by TpeL (and possibly TcdB as well) for cell entry. Furthermore, internalization of CSPG4, PVRL3, FZD2, and FZD7 was observed neither in MEFs nor in Caco-2 cells. FZD2/7, CSPG4, and PVRL3 did thus exhibit no constitutive recycling. The presence of TcdB and the p38 activation induced by anisomycin were not able to induce or enhance CSPG4 or PVRL3 uptake in MEFs. In conclusion, FZD2/7, CSPG4, and PVRL3 seem to serve as cell surface binding receptors rather than internalizing receptors of TcdB.
Keywords: Clostridium perfringens; cell surface; clostridial glycosylating toxins; clostridioides difficile infection; endocytosis; fibroblasts/myofibroblast; human intestinal organoids; receptors.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

