Potential Role of MicroRNAs in the Regulation of Antiviral Responses to Influenza Infection
- PMID: 30022983
- PMCID: PMC6039551
- DOI: 10.3389/fimmu.2018.01541
Potential Role of MicroRNAs in the Regulation of Antiviral Responses to Influenza Infection
Abstract
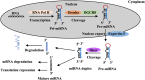
Influenza is a major health burden worldwide and is caused by influenza viruses that are enveloped and negative stranded RNA viruses. Little progress has been achieved in targeted intervention, either at a population level or at an individual level (to treat the cause), due to the toxicity of drugs and ineffective vaccines against influenza viruses. MicroRNAs (miRNAs) are small non-coding RNAs that play critical roles in gene expression, cell differentiation, and tissue development and have been shown to silence viral replication in a sequence-specific manner. Investigation of these small endogenous nucleotides may lead to new therapeutics against influenza virus infection. Here, we describe our current understanding of the role of miRNAs in host defense response against influenza virus, as well as their potential and limitation as new therapeutic approaches.
Keywords: immune responses; infection; inflammation; influenza virus; microRNA.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

