Histopathological and immunohistochemical study of the protective effect of triptorelin on the neurocytes of the hippocampus and the cerebral cortex of male albino rats after short-term exposure to cyclophosphamide
- PMID: 30023218
- PMCID: PMC6014199
- DOI: 10.1016/j.jmau.2015.12.002
Histopathological and immunohistochemical study of the protective effect of triptorelin on the neurocytes of the hippocampus and the cerebral cortex of male albino rats after short-term exposure to cyclophosphamide
Abstract
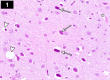
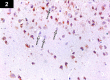
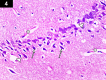
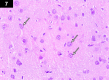
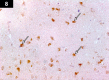
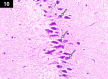
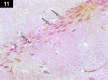
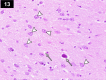
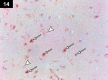
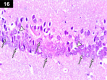
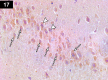
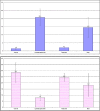
Chemotherapy treats many types of cancer effectively but it often causes side effects. Chemotherapy works on active cells, such as cancer cells, and some healthy cells. Side effects happen when chemotherapy damages these healthy cells. Today, many more drugs are available to treat side effects than in the past. Triptorelin (Decapeptyl) is a gonadotropin-releasing hormone agonist that is reported to have many therapeutic effects besides being an anti-cancer agent. In the current study, intraperitoneal cyclophosphamide (65 mg/kg/day) was administered for 4 weeks to induce marked dystrophic changes in the cerebral cortex and hippocampus of male albino rats. After 4 weeks, we observed significant degeneration of neurocytes with dystrophic changes. Subcutaneous triptorelin (0.05 mg/kg/day) for 4 weeks significantly improved histological signs of degeneration and apoptosis. Anti-Bcl2 staining of sections of the cerebral cortex and hippocampus showed that the apoptotic index was increased. This finding was confirmed by the anti-p53 staining, which showed a significant decrease in the apoptotic index. Ultimately, such improvements were accompanied by significant restoration of normal brain histology, as revealed by hematoxylin and eosin. In conclusion, triptorelin can reverse the apoptotic changes induced by cyclophosphamide therapy, which is more marked in the hippocampus than cerebral cortex.
Keywords: cerebral cortex; cyclophosphamide; hippocampus; p53; triptorelin.
Figures







Similar articles
-
Possible protective effect of royal jelly against cyclophosphamide induced prostatic damage in male albino rats; a biochemical, histological and immuno-histo-chemical study.Biomed Pharmacother. 2017 Jun;90:15-23. doi: 10.1016/j.biopha.2017.03.020. Epub 2017 Mar 21. Biomed Pharmacother. 2017. PMID: 28340377
-
Ameliorating effect of selenium nanoparticles on cyclophosphamide-induced hippocampal neurotoxicity in male rats: light, electron microscopic and immunohistochemical study.Folia Morphol (Warsz). 2021;80(4):806-819. doi: 10.5603/FM.a2020.0117. Epub 2020 Oct 21. Folia Morphol (Warsz). 2021. PMID: 33084015
-
Effect of a gonadotropin-releasing hormone analogue on cyclophosphamide-induced ovarian toxicity in adult mice.Arch Gynecol Obstet. 2013 May;287(5):1023-9. doi: 10.1007/s00404-012-2658-y. Epub 2012 Dec 7. Arch Gynecol Obstet. 2013. PMID: 23224696
-
The model of pentylenetetrazol-induced status epilepticus in the immature rat: short- and long-term effects.Epilepsy Res. 1996 Dec;26(1):93-103. doi: 10.1016/s0920-1211(96)00045-9. Epilepsy Res. 1996. PMID: 8985691
-
Hormonal strategies for fertility preservation in patients receiving cyclophosphamide to treat glomerulonephritis: a nonrandomized trial and review of the literature.Am J Kidney Dis. 2008 Nov;52(5):887-96. doi: 10.1053/j.ajkd.2008.06.017. Am J Kidney Dis. 2008. PMID: 18971013 Review.
Cited by
-
Protective effects of hesperidin in cyclophosphamide-induced parotid toxicity in rats.Sci Rep. 2023 Jan 4;13(1):158. doi: 10.1038/s41598-022-26881-w. Sci Rep. 2023. PMID: 36599902 Free PMC article.
-
Extraction, Phytochemical profile, and neuroprotective activity of Phyllanthus emblica fruit extract against sodium valproate-induced postnatal autism in BALB/c mice.Heliyon. 2024 Jul 20;10(15):e34992. doi: 10.1016/j.heliyon.2024.e34992. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39157403 Free PMC article.
-
Effects of empagliflozin on cyclophosphamide-induced neurotoxicity in rats without diabetes.Turk J Med Sci. 2024 Dec 30;55(1):65-71. doi: 10.55730/1300-0144.5943. eCollection 2025. Turk J Med Sci. 2024. PMID: 40104287 Free PMC article.
-
Sitagliptin Attenuates the Cognitive Deficits in L-Methionine-Induced Vascular Dementia in Rats.Biomed Res Int. 2022 Feb 27;2022:7222590. doi: 10.1155/2022/7222590. eCollection 2022. Biomed Res Int. 2022. PMID: 35265716 Free PMC article.
-
Exercise Normalized the Hippocampal Renin-Angiotensin System and Restored Spatial Memory Function, Neurogenesis, and Blood-Brain Barrier Permeability in the 2K1C-Hypertensive Mouse.Int J Mol Sci. 2022 May 16;23(10):5531. doi: 10.3390/ijms23105531. Int J Mol Sci. 2022. PMID: 35628344 Free PMC article.
References
-
- Wefel JS, Lenzi R, Theriault R, Davis R, Christina AM. The cognitive sequelae of standard dose adjuvant chemotherapy in women with breast cancer: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–9. - PubMed
-
- Alvarez JA, Scully RE, Miller TL, Armstrong FD, Constine LS, Friedman DL, et al. Long-term effects of treatments for childhood cancers. Curr Opin Pediatr. 2007;19:23–31. - PubMed
-
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, et al. Neuropsychological impact of standard dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–93. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
