Elosulfase alfa for mucopolysaccharidosis type IVA: Real-world experience in 7 patients from the Spanish Morquio-A early access program
- PMID: 30023300
- PMCID: PMC6047108
- DOI: 10.1016/j.ymgmr.2018.03.009
Elosulfase alfa for mucopolysaccharidosis type IVA: Real-world experience in 7 patients from the Spanish Morquio-A early access program
Abstract
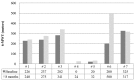
There is a growing interest in evaluating the effectiveness of enzyme replacement therapy (ERT) with elosulfase alfa in patients with mucopolysaccharidosis type IVA (MPS-IVA) under real-world conditions. We present the experience of seven pediatric MPS-IVA patients from the Spanish Morquio-A Early Access Program. Efficacy was evaluated based on the distance walked in the 6-min walking test (6-MWT) and the 3-min-stair-climb-test (3-MSCT) at baseline and after 8 months of ERT treatment. Additionally, urinary glycosaminoglycans were measured, and a molecular analysis of a GALNS mutation was performed. The health-related quality of life was evaluated using the EuroQoL (EQ)-5D-5 L. The distance walked according to the 6-MWT ranged from 0 to 325 m at baseline and increased to 12-300 m after 8 months with elosulfase alfa (the walked distance improved in all patients except one). An increase was observed for the two patients who had to use a wheelchair. Improvements were also observed for the 3-MSCT in four patients, whereas two patients showed no changes. Three patients showed an improvement in the EQ-VAS score, whereas the scores of three patients remained stable. Regarding urinary glycosaminoglycans measurements, an irregular response was observed. Our results showed overall improvement in endurance and functionality after 8 months of elosulfase alfa treatment in a heterogeneous subset of MPS IVA patients with severe clinical manifestations managed in a real-world setting.
Keywords: Elosulfase alfa; Lysosomal storage disorder; MPS IVA; Morquio A; Quality of life; Urinary GAGs.
Figures

References
-
- Chudley A.E., Chakravorty C. Genetic landmarks through philately: Luis Morquio 1867-1935. Clin. Genet. 2002;62(6):438–439. - PubMed
-
- Theroux M.C., Nerker T., Ditro C., Mackenzie W.G. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr. Anaesth. 2012;22(9):901–907. - PubMed
-
- Kakkis E.D., Muenzer J., Tiller G.E. Enzyme-replacement therapy in mucopolysaccharidosis I. New Engl. J. Med. 2001;344(3):182–188. - PubMed
-
- Muenzer J., Wraith J.E., Beck M. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet. Med. 2006;8(8):465–473. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

