Pooled Platelet-Rich Plasma Lysate Therapy Increases Synoviocyte Proliferation and Hyaluronic Acid Production While Protecting Chondrocytes From Synoviocyte-Derived Inflammatory Mediators
- PMID: 30023361
- PMCID: PMC6039577
- DOI: 10.3389/fvets.2018.00150
Pooled Platelet-Rich Plasma Lysate Therapy Increases Synoviocyte Proliferation and Hyaluronic Acid Production While Protecting Chondrocytes From Synoviocyte-Derived Inflammatory Mediators
Abstract
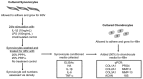
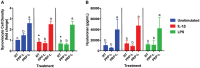
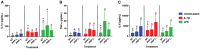
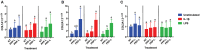
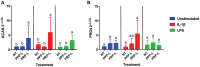
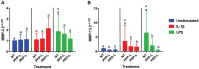
Platelet-rich plasma (PRP) preparations are being used with moderate success to treat osteoarthritis (OA) in humans and in veterinary species. Such preparations are hindered, however, by being autologous in nature and subject to tremendous patient and processing variability. For this reason, there has been increasing interest in the use of platelet lysate preparations instead of traditional PRP. Platelet lysate preparations are acellular, thereby reducing concerns over immunogenicity, and contain high concentrations of growth factors and cytokines. In addition, platelet lysate preparations can be stored frozen for readily available use. The purpose of this study was to evaluate the effects of a pooled allogeneic platelet-rich plasma lysate (PRP-L) preparation on equine synoviocytes and chondrocytes challenged with inflammatory mediators in-vitro to mimic the OA joint environment. Our hypothesis was that PRP-L treatment of inflamed synoviocytes would protect chondrocytes challenged with synoviocyte conditioned media by reducing synoviocyte pro-inflammatory cytokine production while increasing synoviocyte anti-inflammatory cytokine production. Synoviocytes were stimulated with either interleukin-1β (IL-1β) or lipopolysaccharide (LPS) for 24 h followed by no treatment or treatment with platelet-poor plasma lysate (PPP-L) or PRP-L for 48 h. Synoviocyte growth was evaluated at the end of the treatment period and synoviocyte conditioned media was assessed for concentrations of hyaluronic acid (HA), IL-1β, tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6). Chondrocytes were then challenged for 48 h with synoviocyte conditioned media from each stimulation and treatment group and examined for gene expression of collagen types I (COL1A1), II (COL2A1), and III (COL3A1), aggrecan (ACAN), lubricin (PRG4), and matrix metallopeptidase 3 (MMP-3) and 13 (MMP-13). Treatment of inflamed synoviocytes with PRP-L resulted in increased synoviocyte growth and increased synoviocyte HA and IL-6 production. Challenge of chondrocytes with conditioned media from PRP-L treated synoviocytes resulted in increased collagen type II and aggrecan gene expression as well as decreased MMP-13 gene expression. The results of this study support continued investigation into the use of pooled PRP-L for the treatment of osteoarthritis and warrant further in-vitro studies to discern the mechanisms of action of PRP-L.
Keywords: HA; IL-1β; LPS; MMP-13; aggrecan; collagen type II; osteoarthritis; platelet-rich plasma lysate.
Figures
References
-
- Cole BJ, Karas V, Hussey K, Merkow DB, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. (2017) 45:339–46. 10.1177/0363546516665809 - DOI - PubMed
-
- Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. (2017) 12:1–12. 10.1186/s13018-017-0521-3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

