Metal-Organic Framework/Graphene Quantum Dot Nanoparticles Used for Synergistic Chemo- and Photothermal Therapy
- PMID: 30023630
- PMCID: PMC6044744
- DOI: 10.1021/acsomega.6b00385
Metal-Organic Framework/Graphene Quantum Dot Nanoparticles Used for Synergistic Chemo- and Photothermal Therapy
Abstract
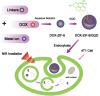
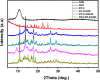
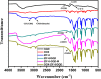
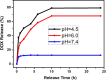
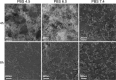
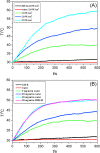
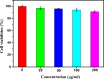
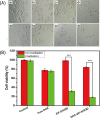
In this study, a simple one-pot method was used to prepare a multifunctional platform for synergistic chemo- and photothermal therapy,, which is composed of zeolitic imidazolate framework-8 (ZIF-8) as drug nanocarriers and the embedded graphene quantum dots (GQDs) as local photothermal seeds. The structure, drug release behavior, photothermal effect, and synergistic therapeutic efficiency of the ZIF-8/GQD nanoparticles were systematically investigated. Using doxorubicin (DOX) as a model anticancer drug, the results showed that monodisperse ZIF-8/GQD nanoparticles with a particle size of 50-100 nm could encapsulate DOX during the synthesis procedure and trigger DOX release under acidic conditions. The DOX-loaded ZIF-8/GQD nanoparticles could efficiently convert near-infrared (NIR) irradiation into heat and thereby increase the temperature. More importantly, with breast cancer 4T1 cells as a model cellular system, the results indicated that the combined chemo- and photothermal therapy with DOX-ZIF-8/GQD nanoparticles exhibited a significant synergistic effect, resulting in a higher efficacy to kill cancer cells compared with chemotherapy and photothermal therapy alone. Hence, ZIF-8/GQD nanoparticles would be promising as versatile nanocarriers for synergistic cancer therapy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Li W.-Z.; Hao X.-L.; Zhao N.; Han W.-X.; Zhai X.-F.; Zhao Q.; Wang Y.-E.; Zhou Y.-Q.; Cheng Y.-C.; Yue Y.-H.; Fu L.-N.; Zhou J.-L.; Wu H.-Y.; Dong C.-J. Propylene glycol-embodying deformable liposomes as a novel drug delivery carrier for vaginal fibrauretine delivery applications. J. Controlled Release 2016, 226, 107–114. 10.1016/j.jconrel.2016.02.024. - DOI - PubMed
-
- Hu X.; Zhang Y.; Zhou H.; Wan H. PEGylated chitosan microspheres as mucoadhesive drug-delivery carriers for puerarin. J. Appl. Polym. Sci. 2015, 132, 42623.10.1002/app.42623. - DOI
-
- Zhang Z.; Tang J.; Liu X.; Shen Y. Synthesis and characterization of phosphate structured dendrimers as drug delivery carriers. Nanomedicine: Nanotechnology, Biology and Medicine 2016, 12, 473.10.1016/j.nano.2015.12.080. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
