Microencapsulation of Live Cells in Synthetic Polymer Capsules
- PMID: 30023677
- PMCID: PMC6044854
- DOI: 10.1021/acsomega.7b00570
Microencapsulation of Live Cells in Synthetic Polymer Capsules
Abstract
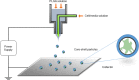
In cell therapies, it is advantageous to encapsulate live cells in protective, semipermeable microparticles for controlled release of cytokines, growth factors, monoclonal antibodies, or insulin. Here, a modified electrospraying approach with an organic solution of poly(lactide-co-glycolide) (PLGA) polymer is used to create synthetic PLGA capsules that effectively protect live cells. Using a design of experiment (DOE) methodology, the effect of governing jetting parameters on encapsulation efficiency, yield, and size is systematically evaluated. On the basis of this analysis, the interaction between bovine serum albumin concentration and core flow rate is the most dominant factor determining core encapsulation efficiency as well as the microcapsule size. However, the interaction between shell solvent ratio and shell flow rate predominantly defines the particle yield. To validate these findings, live cells have been successfully encapsulated in microcapsules using optimized parameters from the DOE analysis and have survived the electrohydrodynamic jetting process. Extending the currently available toolkit for cell microencapsulation, these biodegradable, semi-impermeable cell-laden microcapsules may find a range of applications in areas such as tissue engineering, regenerative medicine, and drug delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Protein encapsulated core-shell structured particles prepared by coaxial electrospraying: investigation on material and processing variables.Int J Pharm. 2014 Oct 1;473(1-2):134-43. doi: 10.1016/j.ijpharm.2014.07.006. Epub 2014 Jul 3. Int J Pharm. 2014. PMID: 24998509
-
Pulsatile protein release from monodisperse liquid-core microcapsules of controllable shell thickness.Pharm Res. 2014 Nov;31(11):3201-10. doi: 10.1007/s11095-014-1412-5. Epub 2014 May 16. Pharm Res. 2014. PMID: 24831313 Free PMC article.
-
Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system.J Control Release. 2010 Oct 15;147(2):193-201. doi: 10.1016/j.jconrel.2010.07.103. Epub 2010 Jul 18. J Control Release. 2010. PMID: 20647022
-
Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications.J Control Release. 2014 Nov 10;193:324-40. doi: 10.1016/j.jconrel.2014.09.003. Epub 2014 Sep 10. J Control Release. 2014. PMID: 25218676 Review.
-
Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives.J Control Release. 2008 Feb 11;125(3):193-209. doi: 10.1016/j.jconrel.2007.09.013. Epub 2007 Oct 22. J Control Release. 2008. PMID: 18083265 Review.
Cited by
-
High Efficiency Vibrational Technology (HEVT) for Cell Encapsulation in Polymeric Microcapsules.Pharmaceutics. 2020 May 21;12(5):469. doi: 10.3390/pharmaceutics12050469. Pharmaceutics. 2020. PMID: 32455714 Free PMC article.
-
Microfluidic Chip Device for In Situ Mixing and Fabrication of Hydrogel Microspheres via Michael-Type Addition.Langmuir. 2021 Oct 12;37(40):11793-11803. doi: 10.1021/acs.langmuir.1c01739. Epub 2021 Oct 1. Langmuir. 2021. PMID: 34597052 Free PMC article.
-
Hierarchical shape-by-shape assembly of microparticles for micrometer-scale viral delivery of two different genes.Biomicrofluidics. 2018 May 4;12(3):031102. doi: 10.1063/1.5030597. eCollection 2018 May. Biomicrofluidics. 2018. PMID: 29774082 Free PMC article.
-
Polymeric Approaches to Reduce Tissue Responses Against Devices Applied for Islet-Cell Encapsulation.Front Bioeng Biotechnol. 2019 Jun 4;7:134. doi: 10.3389/fbioe.2019.00134. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31214587 Free PMC article. Review.
-
Design and Development of Extracellular Matrix Protein-Based Microcapsules as Tools for Bacteria Investigation.Adv Healthc Mater. 2023 Apr;12(11):e2202789. doi: 10.1002/adhm.202202789. Epub 2023 Jan 16. Adv Healthc Mater. 2023. PMID: 36599129 Free PMC article.
References
-
- Sun A. M.; O’Shea G. M. Microencapsulation of living cells — A long-term delivery system. J. Controlled Release 1985, 2, 137–141. 10.1016/0168-3659(85)90039-2. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

