In Vitro Evaluation of Novel Nitazoxanide Derivatives against Mycobacterium tuberculosis
- PMID: 30023755
- PMCID: PMC6044914
- DOI: 10.1021/acsomega.7b00892
In Vitro Evaluation of Novel Nitazoxanide Derivatives against Mycobacterium tuberculosis
Abstract
Nitazoxanide has antiparasitic and antibiotic activities including activity against Mycobacterium tuberculosis. We prepared and evaluated a set of its analogues to determine the structure-activity relationship, and identified several amide- and urea-based analogues with low micromolar activity against M. tuberculosis in vitro. Pharmacokinetics in the rat suggested a path forward to obtain bioavailable compounds. The series had a good microbiological profile with bactericidal activity in vitro against replicating and nonreplicating M. tuberculosis. Analogues had limited activity against other Gram-positive bacteria but no activity against Gram-negative bacteria. Our studies identified the key liability in this series as cytotoxicity. Future work concentrating on identifying the target(s) could assist in removing activity against eukaryotic cells.
Conflict of interest statement
The authors declare the following competing financial interest(s): This work was funded by the Lilly TB Drug Discovery Initiative (http://www.tbdrugdiscovery.org/), and funding was provided by Eli Lilly and Company. The following authors are employed by Eli Lilly & Company: Jeffrey Cramer, Thierry Masquelin, and Philip A. Hipskind, and each were involved in data collection, analysis, and preparation of the manuscript as detailed in the authors’ contribution as part of their normal work.
Figures
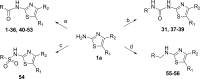
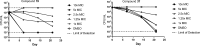
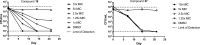
References
-
- World Health Organization . Global Tuberculosis Report 2016. Tuberculosis Fact Sheet No. 104; World Health Organization: Geneva, Switzerland, 2016. http://www.who.int/mediacentre/factsheets/fs104/en/.
LinkOut - more resources
Full Text Sources
Other Literature Sources

