Chemical Profiling of Medical Cannabis Extracts
- PMID: 30023762
- PMCID: PMC6044620
- DOI: 10.1021/acsomega.7b00996
Chemical Profiling of Medical Cannabis Extracts
Abstract
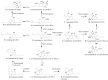
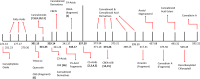
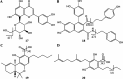
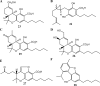
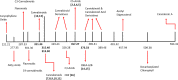
Medical cannabis has been legally available for patients in a number of countries. Licensed producers produce a variety of cannabis strains with different concentrations of phytocannabinoids. Phytocannabinoids in medical cannabis are decarboxylated when subjected to heating for consumption by the patients or when extracted for preparing cannabis derivative products. There is little understanding of the true chemical composition of cannabis extracts, changes occurring during heating of the extracts, and their relevance to pharmacological effects. We investigated the extract from a popular commercial strain of medical cannabis, prior to and after decarboxylation, to understand the chemical profiles. A total of up to 62 compounds could be identified simultaneously in the extract derived from commercial cannabis, including up to 23 phytocannabinoids. Upon heating, several chemical changes take place, including the loss of carboxylic group from the acidic phytocannabinoids. This investigation attempts to reveal the chemical complexity of commercial medical cannabis extracts and the differences in the chemical composition of the native extract and the one subjected to heat. Comprehensive chemical analyses of medical cannabis extracts are needed for standardization, consistency, and, more importantly, an informed employment of this substance for therapeutic purposes.
Conflict of interest statement
The authors declare the following competing financial interest(s): L.P.K. and H.A.C. serve on the scientific and medical advisory board of Scientus Pharma, Inc. and receive a consulting fee.
Figures
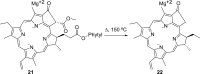
References
-
- Handbook of Cannabis; Pertwee R. G. E., Ed.; Oxford University Press: Oxford, 2014.
-
- Cottone E.; Pomatto V.; Cerri F.; Campantico E.; Mackie K.; Delpero M.; Guastalla A.; Dati C.; Bovolin P.; Franzoni M. F. Cannabinoid receptors are widely expressed in goldfish: molecular cloning of a CB2-like receptor and evaluation of CB1 and CB2 mRNA expression profiles in different organs. Fish Physiol. Biochem. 2013, 39, 1287–1296. 10.1007/s10695-013-9783-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

