Pyridinium-Functionalized Pyromellitic Diimides with Stabilized Radical Anion States
- PMID: 30023774
- PMCID: PMC6045377
- DOI: 10.1021/acsomega.7b01887
Pyridinium-Functionalized Pyromellitic Diimides with Stabilized Radical Anion States
Abstract
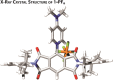
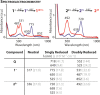
In this work, we report the stabilization of the reduced states of pyromellitic diimide by charge-balancing the imide radical anions with cationic pyridinium groups attached to the aromatic core. This structural modification is confirmed by single-crystal X-ray diffraction analysis. Characterization by (spectro)electrochemical experiments and computations reveal that the addition of cationic groups to an already electron-deficient ring system results in up to +0.57 V shifts in reduction potentials, largely as a consequence of charge screening and lowest unoccupied molecular orbital-lowering effects. This formal charge-balancing approach to stabilizing the reduced states of electron-deficient pyromellitic diimides will facilitate their incorporation into spin-based optoelectronic materials and devices.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Barnes J. C.; Fahrenbach A. C.; Cao D.; Dyar S. M.; Frasconi M.; Giesener M. A.; Benítez D.; Tkatchouk E.; Chernyashevskyy O.; Shin W. H.; Li H.; Sampath S.; Stern C. L.; Sarjeant A. A.; Hartlieb K. J.; Liu Z.; Carmieli R.; Botros Y. Y.; Choi J. W.; Slawin A. M. Z.; Ketterson J. B.; Wasielewski M. R.; Goddard W. A. III; Stoddart J. F. A Radically Configurable Six-State Compound. Science 2013, 339, 429–433. 10.1126/science.1228429. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

