Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase
- PMID: 30023785
- PMCID: PMC6044782
- DOI: 10.1021/acsomega.7b01633
Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase
Abstract
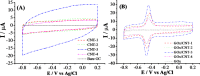
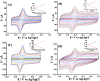
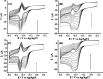
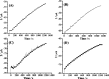
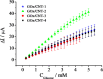
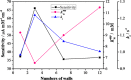
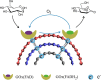
Carbon nanotubes (CNTs) are excellent supports for electrocatalysts because of their large surface area, excellent electronic conductivity, and high chemical and structural stability. In the present study, the activity of CNTs on direct electron transfer (DET) and on immobilized glucose oxidase (GOX) is studied as a function of number of walls of CNTs. The results indicate that the GOX immobilized by the CNTs maintains its electrocatalytic activity toward glucose; however, the DET and electrocatalytic activity of GOX depend strongly on the number of inner tubes of CNTs. The GOX immobilized on triple-walled CNTs (TWNTs) has the highest electron-transfer rate constant, 1.22 s-1, for DET, the highest sensitivity toward glucose detection, 66.11 ± 5.06 μA mM-1 cm-2, and the lowest apparent Michaelis-Menten constant, 6.53 ± 0.58 mM, as compared to GOX immobilized on single-walled and multiwalled CNTs. The promotion effect of CNTs on the GOX electrocatalytic activity and DET is most likely due to the electron-tunneling effect between the outer wall and inner tubes of TWNTs. The results of this study have general implications for the fundamental understanding of the role of CNT supports in DET processes and can be used for the better design of more effective electrocatalysts for biological processes including biofuel cells and biosensors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Das D.; Ghosh S.; Basumallick I. Electrochemical Studies on Glucose Oxidation in an Enzymatic Fuel Cell with Enzyme Immobilized on to Reduced Graphene Oxide Surface. Electroanalysis 2014, 26, 2408–2418. 10.1002/elan.201400245. - DOI
-
- Krikstolaityte V.; Oztekin Y.; Kuliesius J.; Ramanaviciene A.; Yazicigil Z.; Ersoz M.; Okumus A.; Kausaite-Minkstimiene A.; Kilic Z.; Solak A. O.; Makaraviciute A.; Ramanavicius A. Biofuel Cell Based on Anode and Cathode Modified by Glucose Oxidase. Electroanalysis 2013, 25, 2677–2683. 10.1002/elan.201300482. - DOI
-
- Leech D.; Kavanagh P.; Schuhmann W. Enzymatic Fuel Cells: Recent Progress. Electrochim. Acta 2012, 84, 223–234. 10.1016/j.electacta.2012.02.087. - DOI
-
- Liu Y.; Du Y.; Li C. M. Direct Electrochemistry Based Biosensors and Biofuel Cells Enabled with Nanostructured Materials. Electroanalysis 2013, 25, 815–831. 10.1002/elan.201200555. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

