Roles of Hydrogen Bonding in Proton Transfer to κP,κN,κP-N(CH2CH2P i Pr2)2-Ligated Nickel Pincer Complexes
- PMID: 30023906
- PMCID: PMC6045406
- DOI: 10.1021/acsomega.8b00777
Roles of Hydrogen Bonding in Proton Transfer to κP,κN,κP-N(CH2CH2P i Pr2)2-Ligated Nickel Pincer Complexes
Abstract
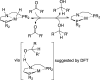
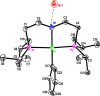
The nickel PNP pincer complex ( i PrPNP)NiPh ( i PrPNP = κP,κN,κP-N(CH2CH2P i Pr2)2) was prepared by reacting ( i PrPNP)NiBr with PhMgCl or deprotonating [( i PrPNHP)NiPh]Y ( i PrPNHP = κP,κN,κP-HN(CH2CH2P i Pr2)2; Y = Br, PF6) with KO t Bu. The byproducts of the PhMgCl reaction were identified as [( i PrPNHP)NiPh]Br and ( i PrPNP')NiPh ( i PrPNP' = κP,κN,κP-N(CH=CHP i Pr2)(CH2CH2P i Pr2)). The methyl analog ( i PrPNP)NiMe was synthesized from the reaction of ( i PrPNP)NiBr with MeLi, although it was contaminated with ( i PrPNP')NiMe due to ligand oxidation. Protonation of ( i PrPNP)NiX (X = Br, Ph, Me) with various acids, such as HCl, water, and MeOH, was studied in C6D6. Nitrogen protonation was shown to be the most favorable process, producing a cationic species [( i PrPNHP)NiX]+ with the NH moiety hydrogen-bonded to the conjugate base (i.e., Cl-, HO-, or MeO-). Protonation of the Ni-C bond was observed at room temperature with ( i PrPNP)NiMe, whereas at 70 °C with ( i PrPNP)NiPh, both resulting in [( i PrPNHP)NiCl]Cl as the final product. Protonation of ( i PrPNP)NiBr was complicated by site exchange between Br- and the conjugate base and by the degradation of the pincer complexes. Indene, which lacks hydrogen-bonding capability, was unable to protonate ( i PrPNP)NiPh and ( i PrPNP)NiMe, despite being more acidic than water and MeOH. Neutral and cationic nickel pincer complexes involved in this study, including ( i PrPNP')NiBr, ( i PrPNP)NiPh, ( i PrPNP')NiPh, ( i PrPNP)NiMe, [( i PrPNHP)NiPh]Y (Y = Br, PF6, BPh4), [( i PrPNHP)NiPh]2[NiCl4], [( i PrPNHP)NiMe]Y (Y = Cl, Br, BPh4), [( i PrPNHP)NiBr]Br, and [( i PrPNHP)NiCl]Cl, were characterized by X-ray crystallography.
Conflict of interest statement
The authors declare no competing financial interest.
Figures














References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

