Arsenic Removal Using "Green" Renewable Feedstock-Based Hydrogels: Current and Future Perspectives
- PMID: 30023930
- PMCID: PMC6044563
- DOI: 10.1021/acsomega.8b00236
Arsenic Removal Using "Green" Renewable Feedstock-Based Hydrogels: Current and Future Perspectives
Abstract
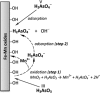
In the recent times, scanty access to clean water has been one of the most prevalent problems, affecting humankind throughout the world. This calls for a tremendous amount of research to recognize new methods of purifying water at lower cost, minimizing the use of hazardous chemicals and impact on the environment. The interest of the scientific community in the potential applications of renewable feedstock-based hydrogels for heavy-metal adsorption for water remediation has been continuously increasing during the last few decades. This study is an effort to highlight the application of hydrogels for revolutionizing the present research on heavy-metal adsorption, particularly arsenic. Besides, the arsenic chemistry, health hazards of arsenic to human health, and adsorption of arsenic by natural polymer-based hydrogels have been reviewed in detail. In addition, challenges in taking the hydrogel technology forward and future prospectives like cost, handling, and disposal of the adsorbent have been discussed systematically.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Hydrogel Adsorbents for the Removal of Hazardous Pollutants-Requirements and Available Functions as Adsorbent.Gels. 2022 Apr 3;8(4):220. doi: 10.3390/gels8040220. Gels. 2022. PMID: 35448121 Free PMC article. Review.
-
Metal-organic frameworks for aquatic arsenic removal.Water Res. 2019 Jul 1;158:370-382. doi: 10.1016/j.watres.2019.04.043. Epub 2019 Apr 24. Water Res. 2019. PMID: 31055017 Review.
-
Iron and aluminium based adsorption strategies for removing arsenic from water.J Environ Manage. 2011 Dec;92(12):3011-22. doi: 10.1016/j.jenvman.2011.07.018. Epub 2011 Aug 25. J Environ Manage. 2011. PMID: 21871703 Review.
-
Recent advances in exploitation of nanomaterial for arsenic removal from water: a review.Nanotechnology. 2017 Jan 27;28(4):042001. doi: 10.1088/1361-6528/28/4/042001. Epub 2016 Dec 20. Nanotechnology. 2017. PMID: 27997365
-
Science and technology for water purification in the coming decades.Nature. 2008 Mar 20;452(7185):301-10. doi: 10.1038/nature06599. Nature. 2008. PMID: 18354474 Review.
Cited by
-
Mesoporous Biopolymer Architecture Enhanced the Adsorption and Selectivity of Aqueous Heavy-Metal Ions.ACS Omega. 2021 May 31;6(23):15316-15331. doi: 10.1021/acsomega.1c01642. eCollection 2021 Jun 15. ACS Omega. 2021. PMID: 34151111 Free PMC article.
-
The Development and Characterization of a Cotton-Chitosan Composite for Lead Removal from Water.Polymers (Basel). 2021 Jun 23;13(13):2066. doi: 10.3390/polym13132066. Polymers (Basel). 2021. PMID: 34201854 Free PMC article.
-
Fe3O4-Functionalized Boron Nitride Nanosheets as Novel Adsorbents for Removal of Arsenic(III) from Contaminated Water.ACS Omega. 2020 Apr 29;5(18):10301-10314. doi: 10.1021/acsomega.9b04295. eCollection 2020 May 12. ACS Omega. 2020. PMID: 32426587 Free PMC article.
-
Multifunctional Cross-Linked Shrimp Waste-Derived Chitosan/MgAl-LDH Composite for Removal of As(V) from Wastewater and Antibacterial Activity.ACS Omega. 2023 Mar 8;8(11):10051-10061. doi: 10.1021/acsomega.2c07391. eCollection 2023 Mar 21. ACS Omega. 2023. PMID: 36969446 Free PMC article.
-
Recent advances of magnetite nanomaterials to remove arsenic from water.RSC Adv. 2022 Nov 9;12(50):32197-32209. doi: 10.1039/d2ra05832d. eCollection 2022 Nov 9. RSC Adv. 2022. PMID: 36425726 Free PMC article. Review.
References
-
- Lee A.; Elam J. W.; Darling S. B. Membrane materials for water purification: design, development, and application. Environ. Sci.: Water Res. Technol. 2016, 2, 17–42. 10.1039/C5EW00159E. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

