Self-Assembly of a Dentinogenic Peptide Hydrogel
- PMID: 30023936
- PMCID: PMC6045409
- DOI: 10.1021/acsomega.8b00347
Self-Assembly of a Dentinogenic Peptide Hydrogel
Abstract
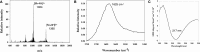
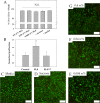
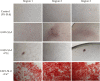
Current standard of care for treating infected dental pulp, root canal therapy, retains the physical properties of the tooth to a large extent, but does not aim to rejuvenate the pulp tissue. Tissue-engineered acellular biomimetic hydrogels have great potential to facilitate the regeneration of the tissue through the recruitment of autologous stem cells. We propose the use of a dentinogenic peptide that self-assembles into β-sheet-based nanofibers that constitute a biodegradable and injectable hydrogel for support of dental pulp stem cells. The peptide backbone contains a β-sheet-forming segment and a matrix extracellular phosphoglycoprotein mimic sequence at the C-terminus. The high epitope presentation of the functional moiety in the self-assembled nanofibers may enable recapitulation of a functional niche for the survival and proliferation of autologous cells. We elucidated the hierarchical self-assembly of the peptide through biophysical techniques, including scanning electron microscopy and atomic force microscopy. The material property of the self-assembled hydrogel was probed though oscillatory rheometry, demonstrating its thixotropic nature. We also demonstrate the cytocompatibility of the hydrogel with respect to fibroblasts and dental pulp stem cells. The self-assembled peptide platform holds promise for guided dentinogenesis and it can be tailored to a variety of applications in soft tissue engineering and translational medicine in the future.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Dye B.; Thornton-Evans G.; Li X.; Iafolla T.. Dental Caries and Tooth Loss in Adults in the United States, 2011–2012. In NCHS Data Brief No. 197; National Center for Health Statistics: Hyattsville, MD, 2015. - PubMed
-
- Gulabivala K.; Ng Y. L.. 1 - Tooth Organogenesis, Morphology and Physiology. In Endodontics, 4th ed.; Mosby, 2014; pp 2–32.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

