Gene transcription in bursting: a unified mode for realizing accuracy and stochasticity
- PMID: 30024089
- PMCID: PMC7379551
- DOI: 10.1111/brv.12452
Gene transcription in bursting: a unified mode for realizing accuracy and stochasticity
Abstract
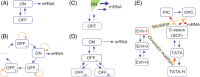
There is accumulating evidence that, from bacteria to mammalian cells, messenger RNAs (mRNAs) are produced in intermittent bursts - a much 'noisier' process than traditionally thought. Based on quantitative measurements at individual promoters, diverse phenomenological models have been proposed for transcriptional bursting. Nevertheless, the underlying molecular mechanisms and significance for cellular signalling remain elusive. Here, we review recent progress, address the above issues and illuminate our viewpoints with simulation results. Despite being widely used in modelling and in interpreting experimental data, the traditional two-state model is far from adequate to describe or infer the molecular basis and stochastic principles of transcription. In bacteria, DNA supercoiling contributes to the bursting of those genes that express at high levels and are topologically constrained in short loops; moreover, low-affinity cis-regulatory elements and unstable protein complexes can play a key role in transcriptional regulation. Integrating data on the architecture, kinetics, and transcriptional input-output function is a promising approach to uncovering the underlying dynamic mechanism. For eukaryotes, distinct bursting features described by the multi-scale and continuum models coincide with those predicted by four theoretically derived principles that govern how the transcription apparatus operates dynamically. This consistency suggests a unified framework for comprehending bursting dynamics at the level of the structural and kinetic basis of transcription. Moreover, the existing models can be unified by a generic model. Remarkably, transcriptional bursting enables regulatory information to be transmitted in a digital manner, with the burst frequency representing the strength of regulatory signals. Such a mode guarantees high fidelity for precise transcriptional regulation and also provides sufficient randomness for realizing cellular heterogeneity.
Keywords: MS2; PP7; WLW model; burst cluster; continuum model; frequency code; gene expression; multi-scale model; ratchet model; temporal occupancy rate.
© 2018 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures





References
-
- Albayrak, C. , Jordi, C. A. , Zechner, C. , Lin, J. , Bichsel, C. A. , Khammash, M. & Tay, S. (2016). Digital quantification of proteins and mRNA in single mammalian cells. Molecular Cell 61, 914–924. - PubMed
-
- Battich, N. , Stoeger, T. & Pelkmans, L. (2013). Image‐based transcriptomics in thousands of single human cells at single‐molecule resolution. Nature Methods 10, 1127–1133. - PubMed
Grants and funding
- 2013CB834104/Ministry of Science and Technology of the People's Republic of China
- JUSRP11838/Fundamental Research Funds for the Central Universities
- 31361163003/National Natural Science Foundation of China
- 81421091/National Natural Science Foundation of China
- 11175084/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Other Literature Sources

