ASK1 contributes to fibrosis and dysfunction in models of kidney disease
- PMID: 30024858
- PMCID: PMC6159961
- DOI: 10.1172/JCI99768
ASK1 contributes to fibrosis and dysfunction in models of kidney disease
Abstract
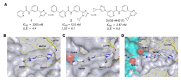
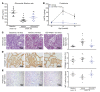
Oxidative stress is an underlying component of acute and chronic kidney disease. Apoptosis signal-regulating kinase 1 (ASK1) is a widely expressed redox-sensitive serine threonine kinase that activates p38 and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase kinases, and induces apoptotic, inflammatory, and fibrotic signaling in settings of oxidative stress. We describe the discovery and characterization of a potent and selective small-molecule inhibitor of ASK1, GS-444217, and demonstrate the therapeutic potential of ASK1 inhibition to reduce kidney injury and fibrosis. Activation of the ASK1 pathway in glomerular and tubular compartments was confirmed in renal biopsies from patients with diabetic kidney disease (DKD) and was decreased by GS-444217 in several rodent models of kidney injury and fibrosis that collectively represented the hallmarks of DKD pathology. Treatment with GS-444217 reduced progressive inflammation and fibrosis in the kidney and halted glomerular filtration rate decline. Combination of GS-444217 with enalapril, an angiotensin-converting enzyme inhibitor, led to a greater reduction in proteinuria and regression of glomerulosclerosis. These results identify ASK1 as an important target for renal disease and support the clinical development of an ASK1 inhibitor for the treatment of DKD.
Keywords: Apoptosis; Chronic kidney disease; Fibrosis; Nephrology; Therapeutics.
Conflict of interest statement
Figures






Comment in
-
ASK1 inhibition shows potential in DKD.Nat Rev Nephrol. 2018 Nov;14(11):658. doi: 10.1038/s41581-018-0063-x. Nat Rev Nephrol. 2018. PMID: 30237443 No abstract available.
-
Targeting oxidative stress in diabetic kidney disease: a novel drug in an old pathway.Kidney Int. 2018 Dec;94(6):1038-1039. doi: 10.1016/j.kint.2018.10.004. Kidney Int. 2018. PMID: 30466558 No abstract available.
References
-
- Evans M, Bain SC, Hogan S, Bilous RW, Collaborative Study Group participants Irbesartan delays progression of nephropathy as measured by estimated glomerular filtration rate: post hoc analysis of the Irbesartan Diabetic Nephropathy Trial. Nephrol Dial Transplant. 2012;27(6):2255–2263. doi: 10.1093/ndt/gfr696. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

