Th1 memory differentiates recombinant from live herpes zoster vaccines
- PMID: 30024861
- PMCID: PMC6159998
- DOI: 10.1172/JCI121484
Th1 memory differentiates recombinant from live herpes zoster vaccines
Abstract
The adjuvanted varicella-zoster virus (VZV) glycoprotein E (gE) subunit herpes zoster vaccine (HZ/su) confers higher protection against HZ than the live attenuated zoster vaccine (ZV). To understand the immunologic basis for the different efficacies of the vaccines, we compared immune responses to the vaccines in adults 50 to 85 years old. gE-specific T cells were very low/undetectable before vaccination when analyzed by FluoroSpot and flow cytometry. Both ZV and HZ/su increased gE-specific responses, but at peak memory response (PMR) after vaccination (30 days after ZV or after the second dose of HZ/su), gE-specific CD4+ and CD8+ T cell responses were 10-fold or more higher in HZ/su compared with ZV recipients. Comparing the vaccines, T cell memory responses, including gE-IL-2+ and VZV-IL-2+ spot-forming cells (SFCs), were higher in HZ/su recipients and cytotoxic and effector responses were lower. At 1 year after vaccination, all gE-Th1 and VZV-IL-2+ SFCs remained higher in HZ/su compared with ZV recipients. Mediation analyses showed that IL-2+ PMR were necessary for the persistence of Th1 responses to either vaccine and VZV-IL-2+ PMR explained 73% of the total effect of HZ/su on persistence. This emphasizes the biological importance of the memory responses, which were clearly superior in HZ/su compared with ZV participants.
Keywords: Adaptive immunity; Infectious disease; Vaccines.
Conflict of interest statement
Figures

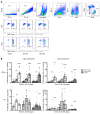
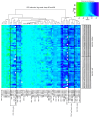
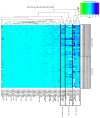

Comment in
-
Tale of two vaccines: differences in response to herpes zoster vaccines.J Clin Invest. 2018 Oct 1;128(10):4245-4247. doi: 10.1172/JCI123217. Epub 2018 Sep 4. J Clin Invest. 2018. PMID: 30179221 Free PMC article.
References
-
- Levin MJ. Zoster vaccines. In: Plotkin S, Orenstein W, Offitt P, Edwards KM, ed. Plotkin’s vaccines. 7th E. Philadelphia, PA: Elsevier; 2017:1268–1281.
-
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

