Spatial Patterns and Age-Related Changes of the Collagen Crimp in the Human Cornea and Sclera
- PMID: 30025116
- PMCID: PMC5995484
- DOI: 10.1167/iovs.17-23474
Spatial Patterns and Age-Related Changes of the Collagen Crimp in the Human Cornea and Sclera
Abstract
Purpose: Collagen is the main load-bearing component of the eye, and collagen crimp is a critical determinant of tissue mechanical behavior. We test the hypothesis that collagen crimp morphology varies over the human cornea and sclera and with age.
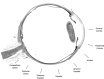
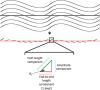
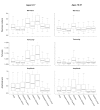
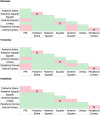
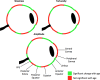
Methods: We analyzed 42 axial whole-globe sections from 20 normal eyes of 20 human donors, ranging in age from 0.08 (1 month) to 97 years. The sections were imaged using polarized light microscopy to obtain μm-scale fiber bundle/lamellae orientation from two corneal and six scleral regions. Crimp morphology was quantified through waviness, tortuosity, and amplitude.
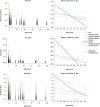
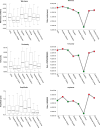
Results: Whole-globe median waviness, tortuosity, and amplitude were 0.127 radians, 1.002, and 0.273 μm, respectively. These parameters, however, were not uniform over the globe, instead exhibiting distinct, consistent patterns. All crimp parameters decreased significantly with age, with significantly different age-related decreases between regions. The crimp morphology of the limbus changed the most drastically with age, such that it had the largest crimp in neonates, and among the smallest in the elderly.
Conclusions: Age-related decreases in crimp parameters are likely one of the mechanisms underlying age-related stiffening of the sclera and cornea, potentially influencing sensitivity to IOP. Further work is needed to determine the biomechanical implications of the crimp patterns observed. The comparatively large changes in the crimp morphology of the limbus, especially in the early years of life, suggest that crimp in this region may play a role in eye development, although the exact nature of this is unclear.
Figures


References
-
- Fratzl P. . Collagen: Structure and Mechanics. Boston, MA: Springer Science & Business Media; 2008.
-
- Standring S. . Gray's Anatomy: The Anatomical Basis of Clinical Practice. 41st ed. New York: Elsevier Limited; 2016.
-
- Andreo RH, Farrell RA. . Corneal small-angle light-scattering theory: wavy fibril models. J Opt Soc Am. 1982; 72: 1479– 1492. - PubMed
-
- Gallagher B, Maurice D. . Striations of light scattering in the corneal stroma. J Ultrastruct Res. 1977; 61: 100– 114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

