Clinical, biochemical and genetic spectrum of 70 patients with ACAD9 deficiency: is riboflavin supplementation effective?
- PMID: 30025539
- PMCID: PMC6053715
- DOI: 10.1186/s13023-018-0784-8
Clinical, biochemical and genetic spectrum of 70 patients with ACAD9 deficiency: is riboflavin supplementation effective?
Abstract
Background: Mitochondrial acyl-CoA dehydrogenase family member 9 (ACAD9) is essential for the assembly of mitochondrial respiratory chain complex I. Disease causing biallelic variants in ACAD9 have been reported in individuals presenting with lactic acidosis and cardiomyopathy.
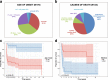
Results: We describe the genetic, clinical and biochemical findings in a cohort of 70 patients, of whom 29 previously unpublished. We found 34 known and 18 previously unreported variants in ACAD9. No patients harbored biallelic loss of function mutations, indicating that this combination is unlikely to be compatible with life. Causal pathogenic variants were distributed throughout the entire gene, and there was no obvious genotype-phenotype correlation. Most of the patients presented in the first year of life. For this subgroup the survival was poor (50% not surviving the first 2 years) comparing to patients with a later presentation (more than 90% surviving 10 years). The most common clinical findings were cardiomyopathy (85%), muscular weakness (75%) and exercise intolerance (72%). Interestingly, severe intellectual deficits were only reported in one patient and severe developmental delays in four patients. More than 70% of the patients were able to perform the same activities of daily living when compared to peers.
Conclusions: Our data show that riboflavin treatment improves complex I activity in the majority of patient-derived fibroblasts tested. This effect was also reported for most of the treated patients and is mirrored in the survival data. In the patient group with disease-onset below 1 year of age, we observed a statistically-significant better survival for patients treated with riboflavin.
Keywords: Activities of daily living; Cardiomyopathy; Complex I; Heart transplantation; Lactic acidosis; Mitochondrial disorder; Neonatal; Prognosis; Treatment; Vitamin.
Conflict of interest statement
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Consent for publication
Written informed consent was obtained from all individuals or caregivers.
Competing interests
All authors declare no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Sánchez-Caballero L, Guerrero-Castillo S, Nijtmans L. Unraveling the complexity of mitochondrial complex I assembly: a dynamic process. BBA - Bioenerg. 1857;2016:980–990. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 2016YFC1306203/National Key Research and Developmental Program of China/International
- 01GM1603/German Bundesministerium für Bildung und Forschung (BMBF) and Horizon2020 through the -RARE (GENOMIT Project)/International
- MC_UU_00015/5/MRC_/Medical Research Council/United Kingdom
- I 2741/FWF_/Austrian Science Fund FWF/Austria
- 203105/Z/16/Z/Wellcome Centre for Mitochondrial Research/International
- doctoral fellowship NIHR-HCS-D12-03-04/National Institute for Health Research/International
- MR/J010448/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00015/8/MRC_/Medical Research Council/United Kingdom
- R01 DK078775/DK/NIDDK NIH HHS/United States
- NIH R01 DK78775/NH/NIH HHS/United States
- 317433/EU FP7 Mitochondrial European Educational Training Project/International
- G0800674/MRC_/Medical Research Council/United Kingdom
- MC_UP_1002/1/MRC_/Medical Research Council/United Kingdom
- 633974/Horizon 2020 (SOUND)/International
- 01GM1207/German Bundesministerium für Bildung und Forschung (BMBF) and Horizon2020 through the E-RARE (GENOMIT Project)/International
- FWF I 2741-B26/German Bundesministerium für Bildung und Forschung (BMBF) and Horizon2020 through the E-RARE (GENOMIT Project)/International
- EXC 115/Cluster of Excellence "Macromolecular Complexes" at the Goethe University Frankfurt/International
- 317433/FP7 Ideas: European Research Council/International
- G0800674/Medical Research Council (MRC) Centre for Translational Research in Neuromuscular Disease, Mitochondrial Disease Patient Cohort (UK)/International
- WT_/Wellcome Trust/United Kingdom
- 01GM1113A/B/C/D/BMBF (mitoNET)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

